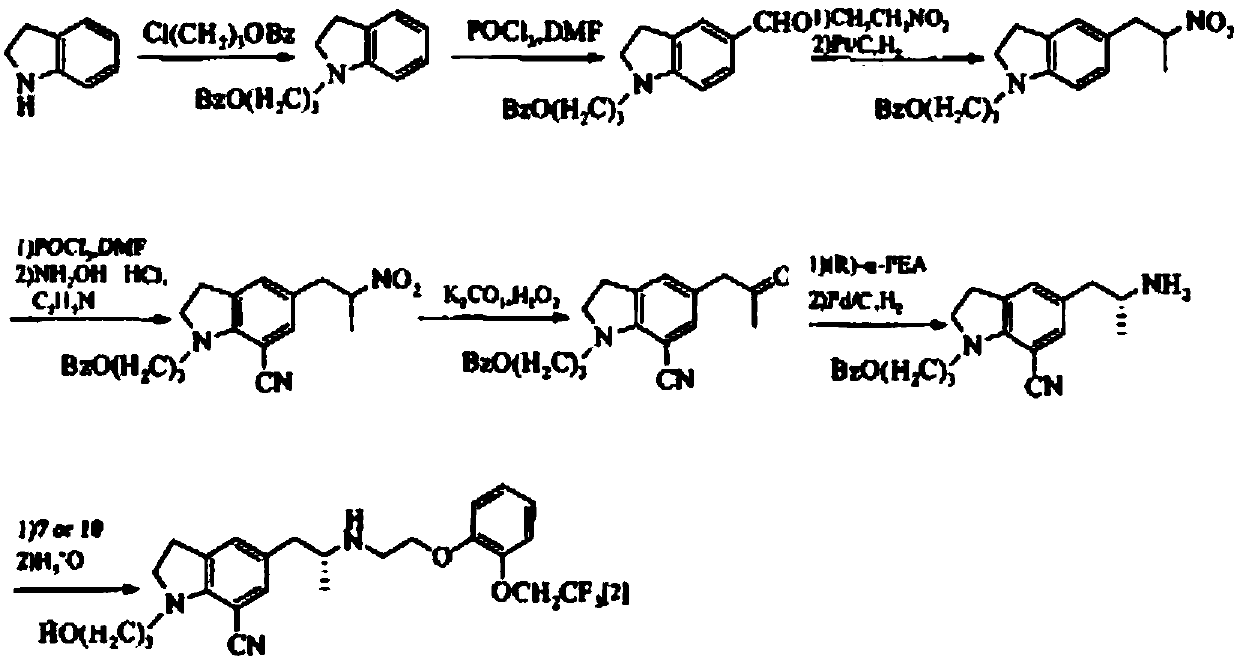
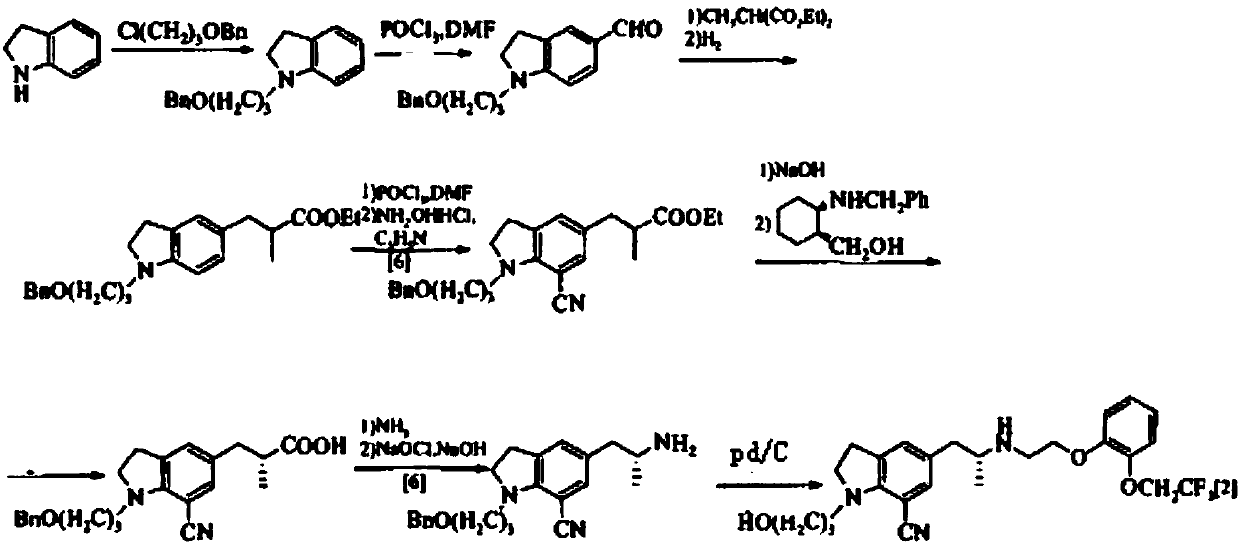
Preparation method of silodosin intermediate
A technology for silodosin and intermediates, which is applied in the field of medicinal chemistry, can solve the problems of many synthesis steps, low total yield, and large environmental impact, and achieve the effects of simple post-processing, good product quality, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
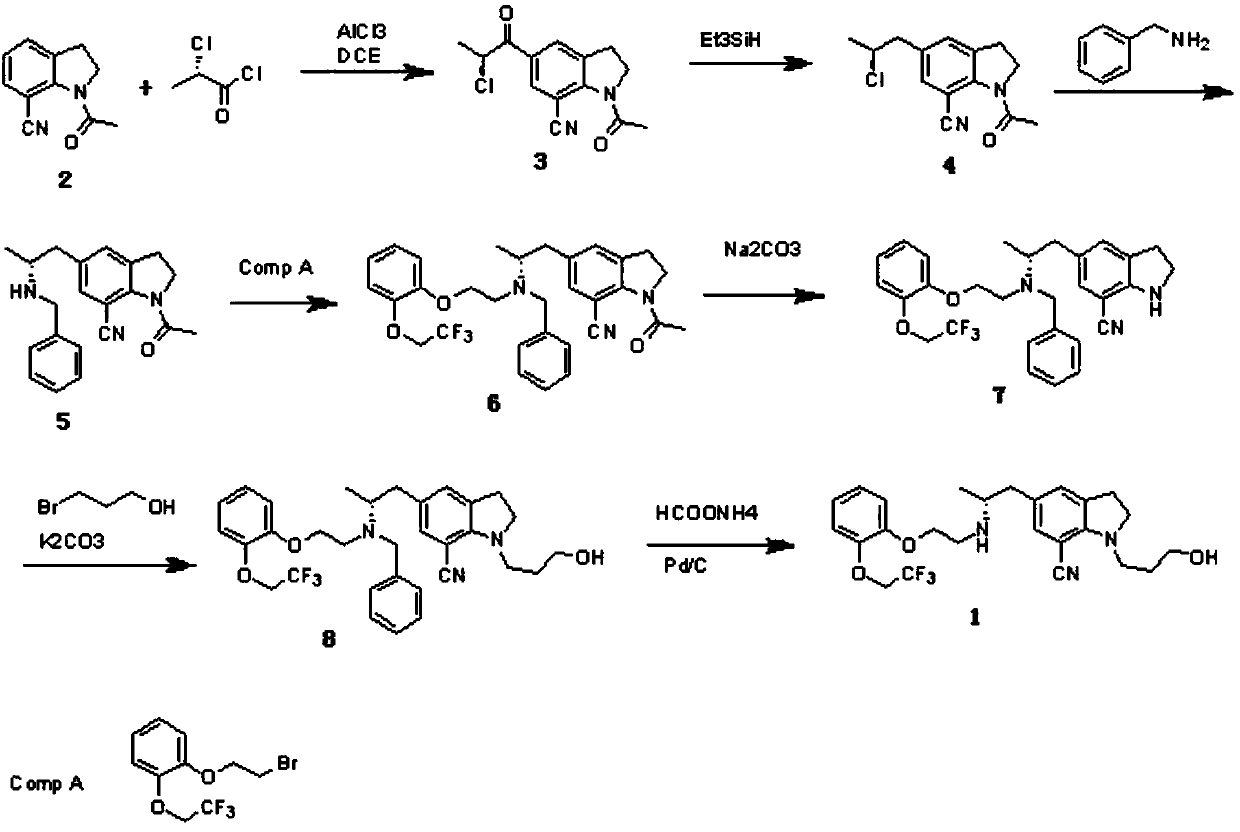
[0038] Embodiment 1: the preparation of compound 3
[0039] Add 460ml of dichloroethane and 36.8g of anhydrous aluminum trichloride into the flask, cool to 0-5°C, and stir for 1h; then add 37.2g of 7-cyanoindoline, and stir for 10min; 30.5g of S-3 Dissolve -chloropropionyl chloride in 150ml of dichloroethane, slowly drop it into the flask from the dropping funnel, keep at 0-5°C, stir for 5h, then react overnight at 20°C; slowly pour the reactant into crushed ice , stirred for 2h; separated the organic layer, washed with water, washed with saturated sodium bicarbonate, and then washed with water until nearly neutral; concentrated under reduced pressure to obtain a light brown solid; added 50ml of ethyl acetate to reflux and stirred, cooled to 20°C, and suction filtered to obtain off-white Solid 48.3g, yield 87.5%.
Embodiment 2
[0040] Embodiment 2: the preparation of compound 4
[0041] Dissolve 41.4g of compound 3 in 270ml of trifluoroacetic acid, cool down to 5°C in an ice bath, slowly add 25.94g of triethylsilane dropwise, keep at 5-10°C, drop it for about 0.5h; keep stirring for 3h, evaporate under reduced pressure solvent, washed off the supernatant with n-hexane; the residue was dissolved in 200ml of dichloromethane, washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate and distilled off the dichloromethane to obtain 36.4g of dark brown liquid, yield 92.7%.
Embodiment 3
[0042] Embodiment 3: the preparation of compound 5
[0043] 260ml of acetonitrile, 39.4g of compound 4, 19.3g of benzylamine and 18.2g of triethylamine were added into the flask, and the temperature was raised to 50°C to react overnight. Evaporate the solvent under reduced pressure, add 220ml of dichloromethane and 350ml of water, stir for 1h; separate the organic layer, concentrate under reduced pressure to an oily substance; add 300ml of hydrochloric acid (1:1) and stir for 1h, and suction filter to obtain a brown solid; Stir in 500ml of water, slowly add sodium carbonate solution until neutral, stir for 1h, then add sodium carbonate solution until neutral, stir for another 2h; filter with suction, recrystallize the solid with ethanol; get 40.6g of brown solid, yield 81.3 %.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com