Method for preparing and purifying Silodosin intermediates
A purification method and compound technology, which is applied in the direction of ether preparation, sulfonate ester preparation, ester reaction preparation of ether, etc., can solve the problems of long route, low yield, lack of yield, etc., and achieve simple reaction operation and high total yield , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
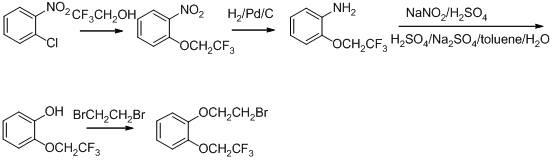
[0019] Example 1 Synthesis of 2-(2,2,2-trifluoroethoxy)phenol (II)
[0020] 100.0g (0.91mol) of o-diphenol, 112.0g (0.41mol) of p-toluenesulfonic acid-2,2,2-trifluoroethyl ester, 50.8g (0.91mol) of potassium hydroxide, 1.0g (6.7 mmol) and 100ml DMF were mixed, stirred at 100°C for 8 hours, poured the reaction solution into 800ml ice water, adjusted the pH value to about 4 with hydrochloric acid, extracted 3 times with 400ml dichloromethane respectively, combined the dichloromethane layers, and decompressed The dichloromethane was evaporated, the residue was distilled with water steam, the fraction was extracted with dichloromethane, the organic layer was dried over anhydrous sodium sulfate, filtered, and the dichloromethane was evaporated under reduced pressure to obtain 52.0 g of a white solid with a yield of 66%, melting point 50-51°C.
Embodiment 2
[0021] Example 2 Synthesis of 2-(2,2,2,-trifluoroethoxy)phenoxyethyl methanesulfonate (I)
[0022] Mix 100g (0.52mol) of 2-(2,2,2-trifluoroethoxy)phenol, 490g (1.56mol) of ethylene dimesylate, and 100g (0.75mol) of potassium carbonate in 1200ml of acetonitrile, After reflux and stirring for 5 hours, the solvent was evaporated under reduced pressure, and 2500 ml of dichloromethane was added to dissolve the residue, which was washed three times with 500 ml of water respectively. The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to evaporate the dichloromethane. The residue was recrystallized with ethyl acetate:petroleum ether (1:50), filtered, and the resulting solid was recrystallized with absolute ethanol, the precipitated solid was discarded, and the filtrate was concentrated, then purified with ethyl acetate:petroleum ether (1:50). 50) Recrystallize, filter, and dry to obtain 85 g of solid, with a yield of 53% and a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com