Novel intermediate for synthesizing silodosin as well as preparation method and purpose of novel intermediate
A technology of intermediates and compounds, which is applied in the field of preparation of intermediate compounds, can solve problems such as high cost and complicated preparation process, and achieve the effects of reduced production costs, controllable optical purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
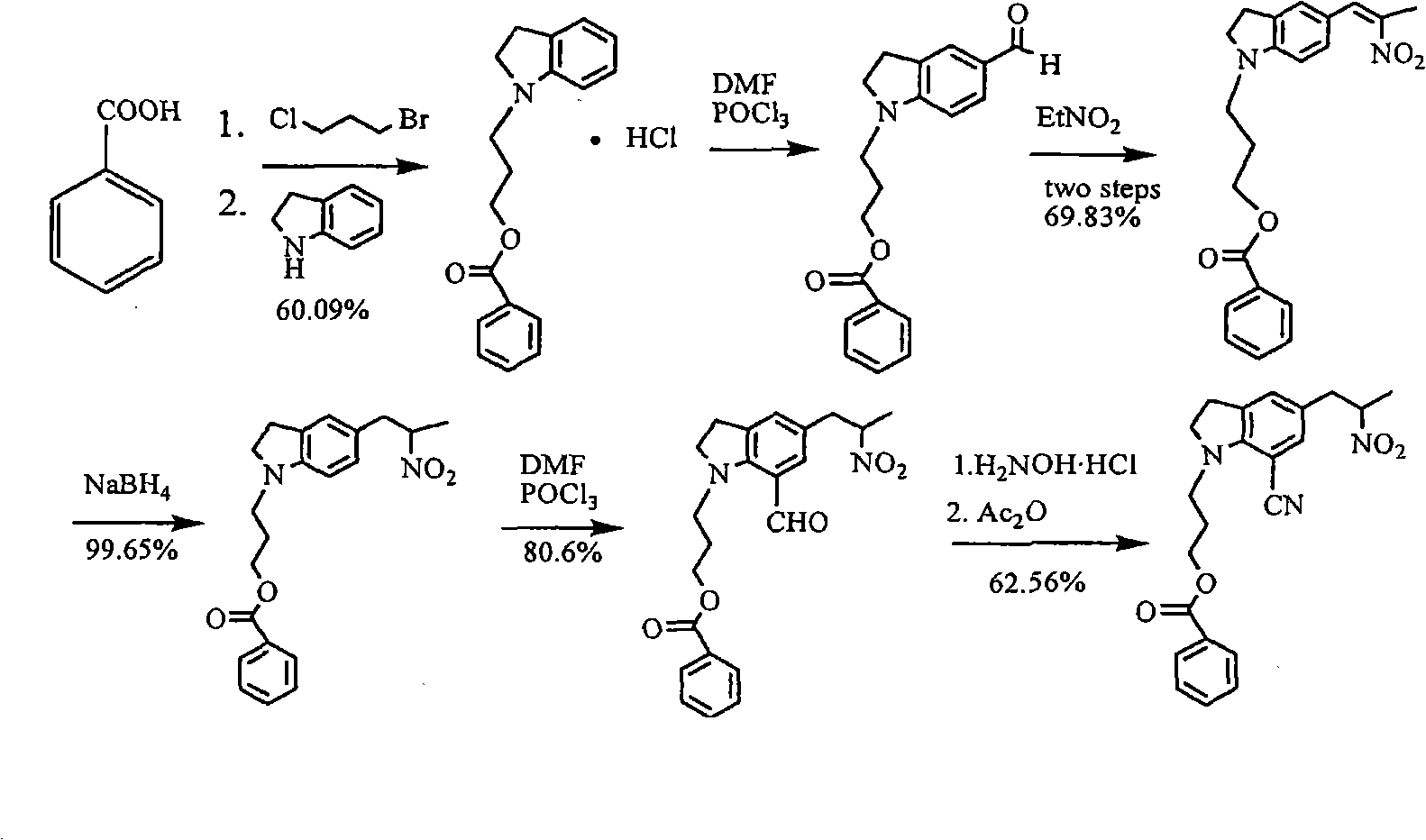
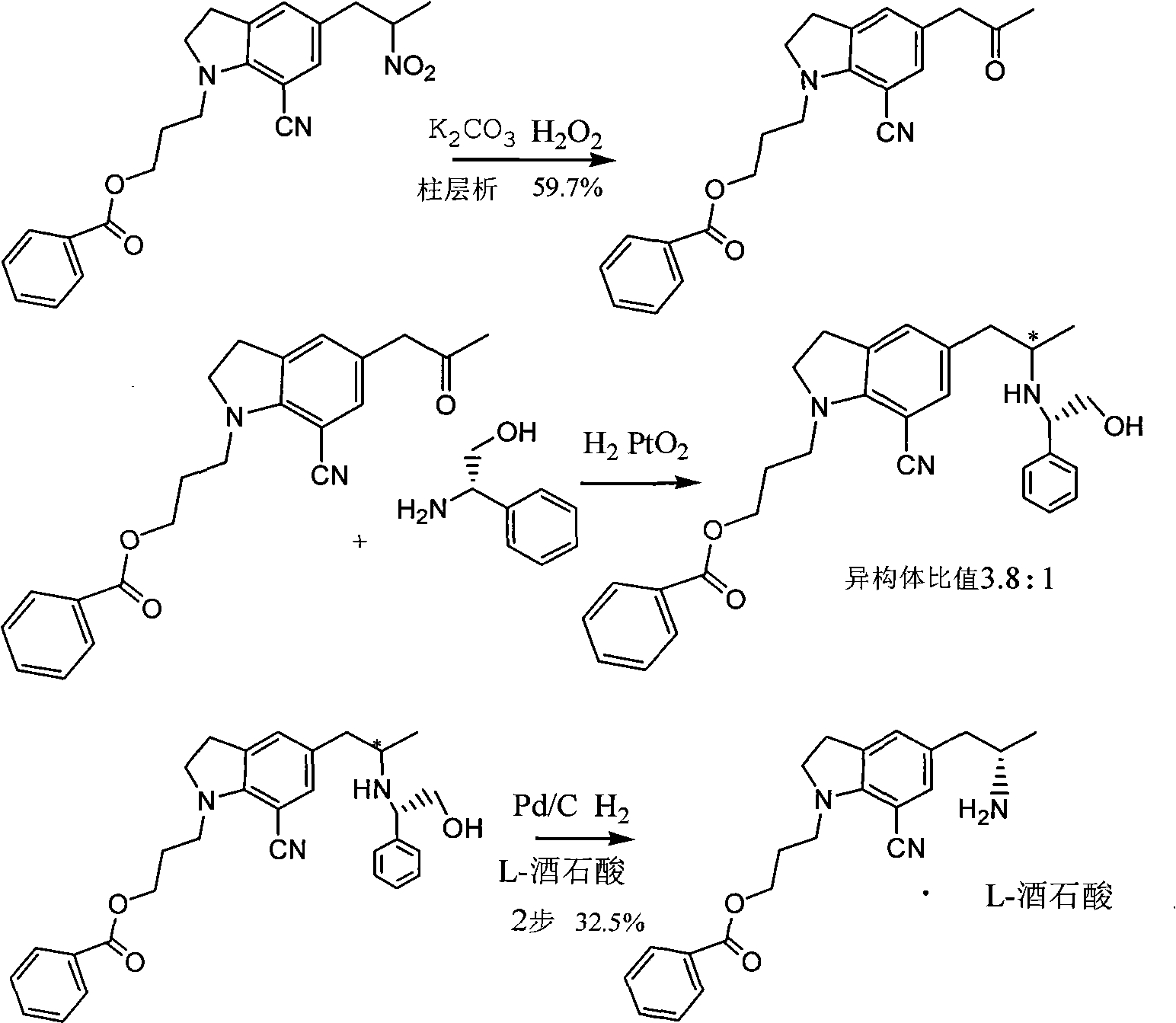
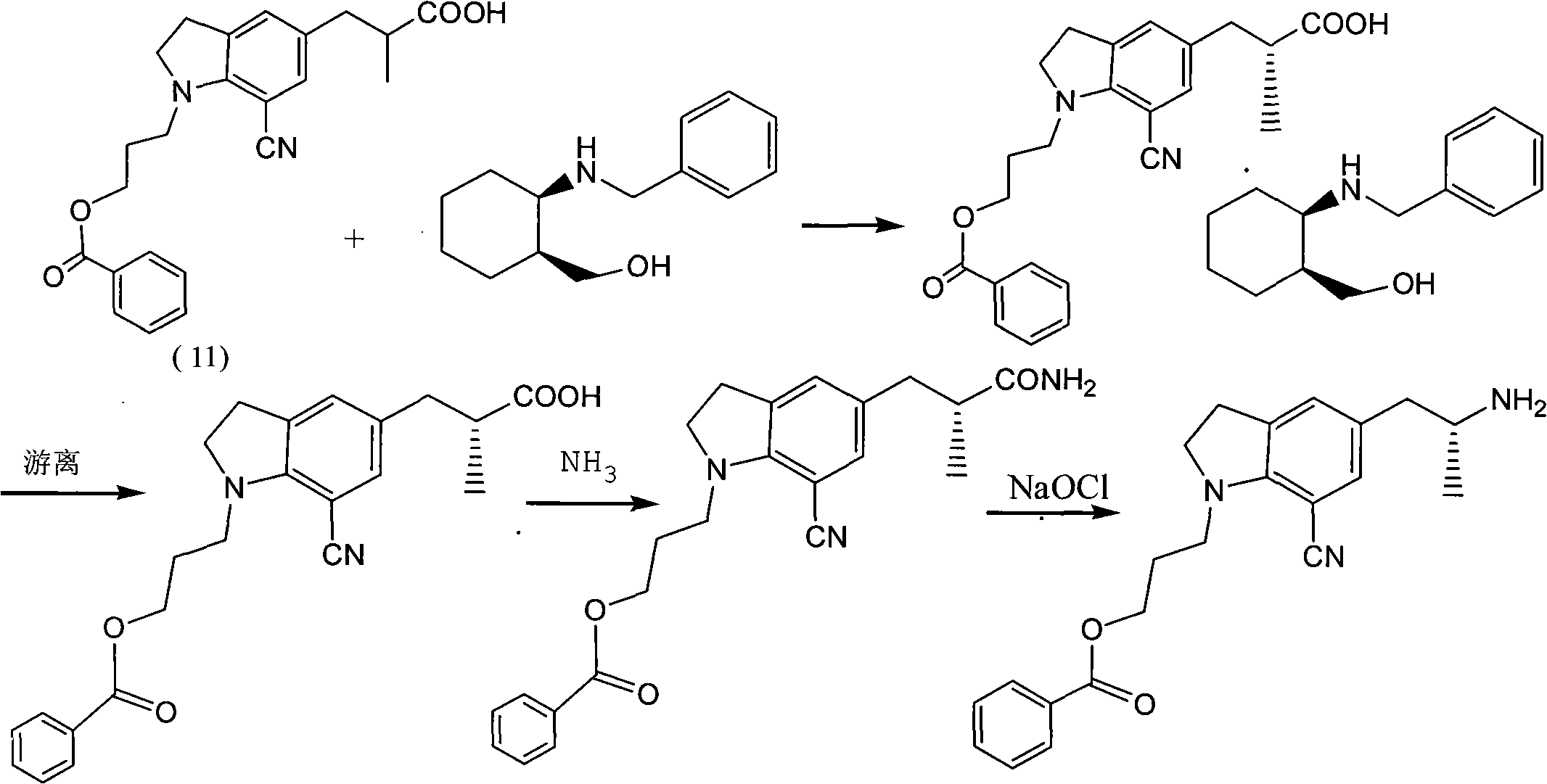
[0032] Example 1 Preparation of 5-[2-(amino)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanindoline L-tartrate
[0033] Add 3 kg of methanol into the hydrogenation kettle, then add 152.4 g of glacial acetic acid, 500 g of 5-[2-(nitro)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanoindol Indoline, 50g of 10% palladium on carbon, after nitrogen replacement, heat to 60-65°C, feed hydrogen to 4-5 atmospheric pressure, react for 12 hours, monitor the reaction by TLC, after the reaction is completed, cool to room temperature, filter to remove palladium on carbon, a little methanol Rinse the palladium carbon, and the filtrate is concentrated under reduced pressure to remove the solvent. With stirring, it is added to the potassium carbonate solution prepared by 525.7 g of potassium carbonate and 2 kg of water, and then 1500 ml of tetrahydrofuran is added, and the organic phase is added by 209.7 g The solution prepared by L-tartaric acid and 1258 grams of water was cooled to about zero degree, kept fo...
Embodiment 2 6
[0037] The preparation of embodiment two to six 5-[2-(amino)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanoindoline L-tartrate
[0038] Add methanol to the hydrogenation kettle, then add glacial acetic acid, 5-[2-(nitro)propyl]-1-[3-(benzoyloxy)propyl]-7-cyanindoline, 10 % palladium on carbon, after nitrogen replacement, heat to 60-65°C, feed hydrogen to 4-5 atmospheric pressure, react for 12 hours, monitor the reaction by TLC, after the reaction is completed, cool to room temperature, remove palladium carbon by filtration, rinse palladium carbon with a little methanol , the filtrate was concentrated under reduced pressure and evaporated to remove the solvent. Under stirring, it was added to the prepared aqueous potassium carbonate solution, then extracted with tetrahydrofuran, separated, the organic phase was added to the pre-prepared L-tartaric acid aqueous solution, cooled to about zero, and kept for 3 Hours, the solid was filtered and weighed after drying.
[0039] The specific ...
Embodiment 2 6
[0040] Table 1 embodiment two to six preparation 5-[2-(amino) propyl]-1-[3-(benzoyloxy) propyl]-7-cyanoindoline L-tartrate feed amount and results
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com