Preparation method for 1-acetyl-7-cyanopyridine-5-(2-amino propyl) indoline
An aminopropyl and acetyl technology, which is applied in the field of preparation of 1-acetyl-7-cyano-5-(2-aminopropyl) indoline, can solve the problem of poor reaction selectivity in the bromination step, industrial The problems of low value and difficult purification can achieve the effect of strong industrial application value, low implementation cost and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
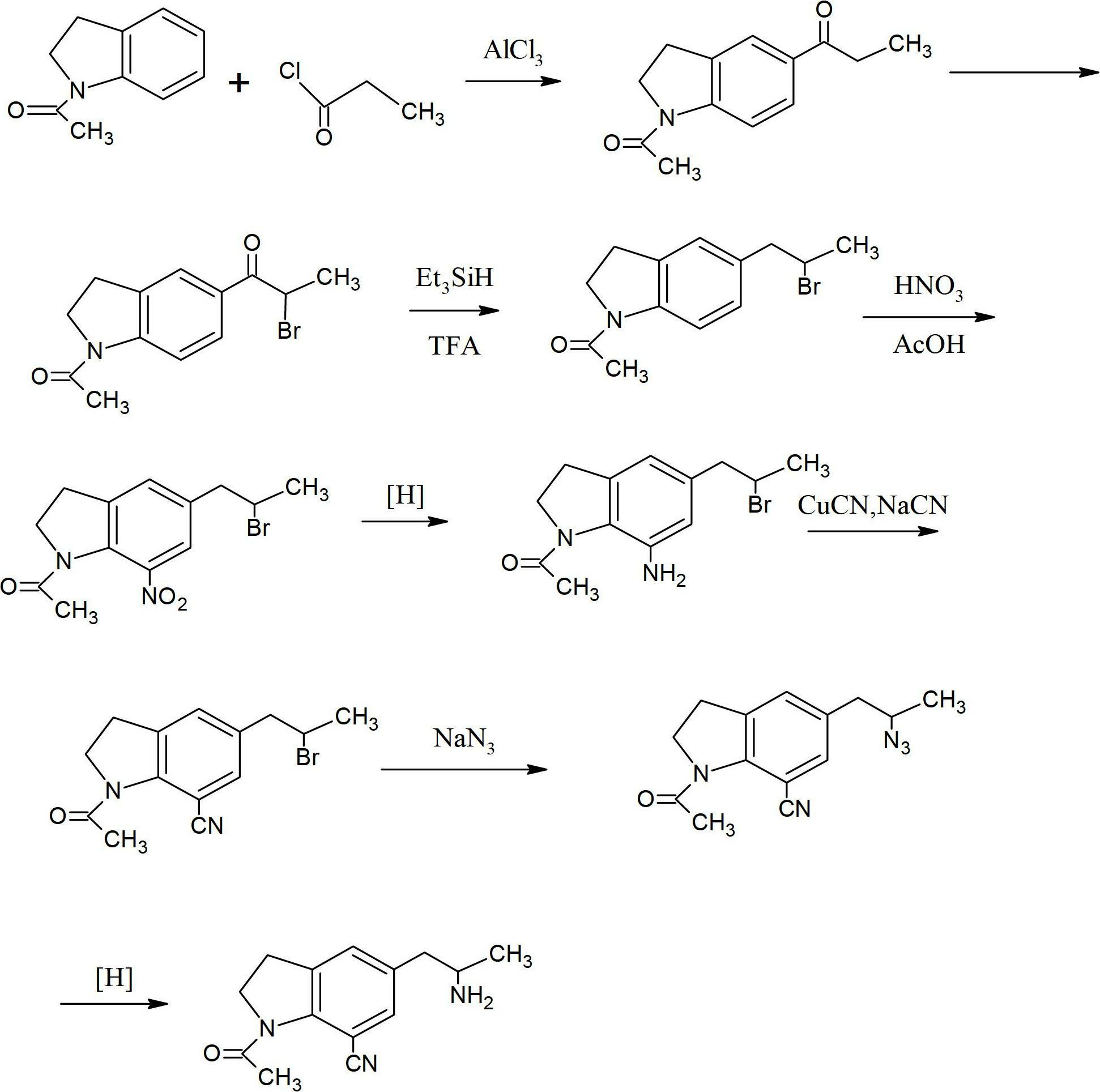
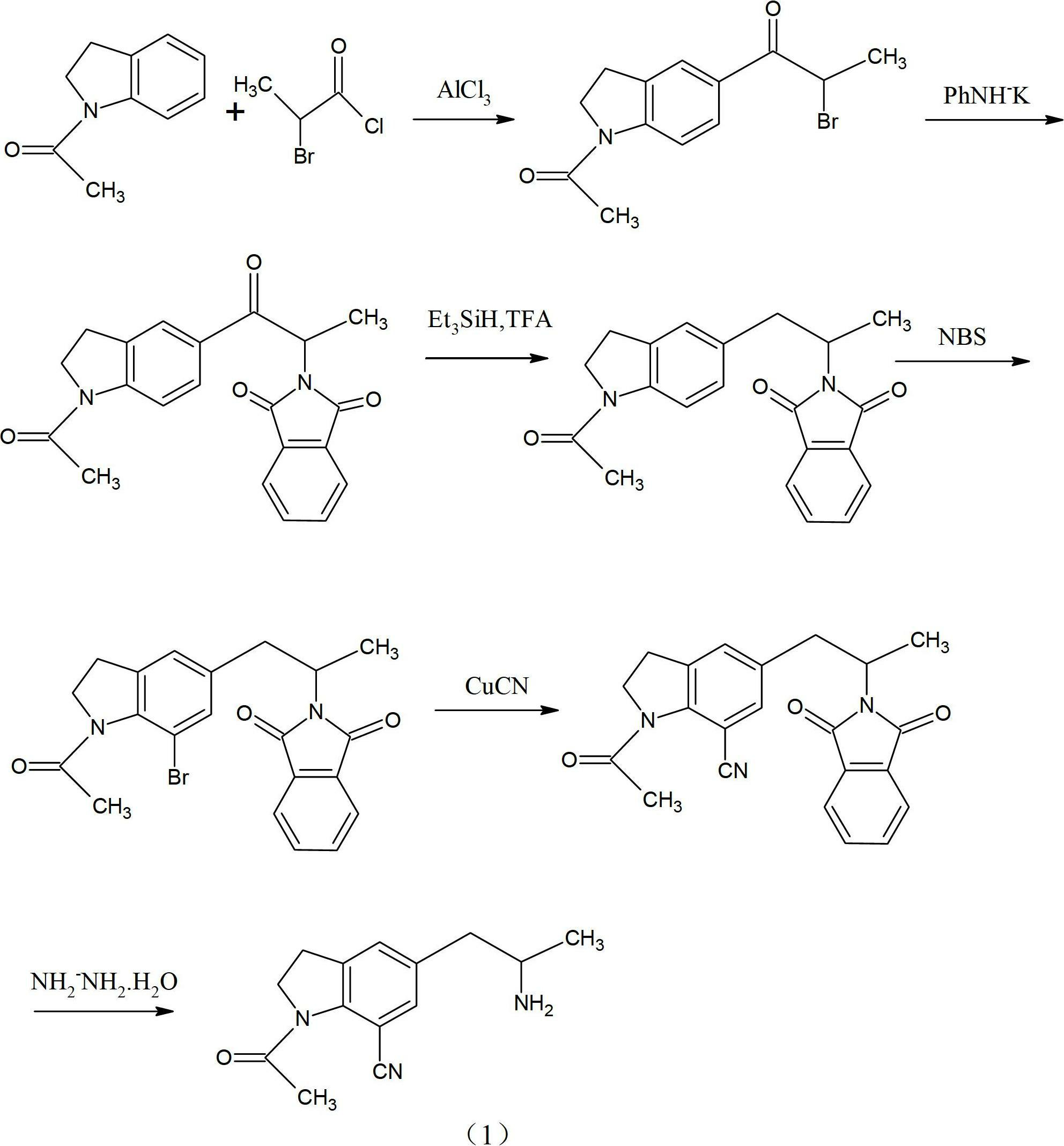
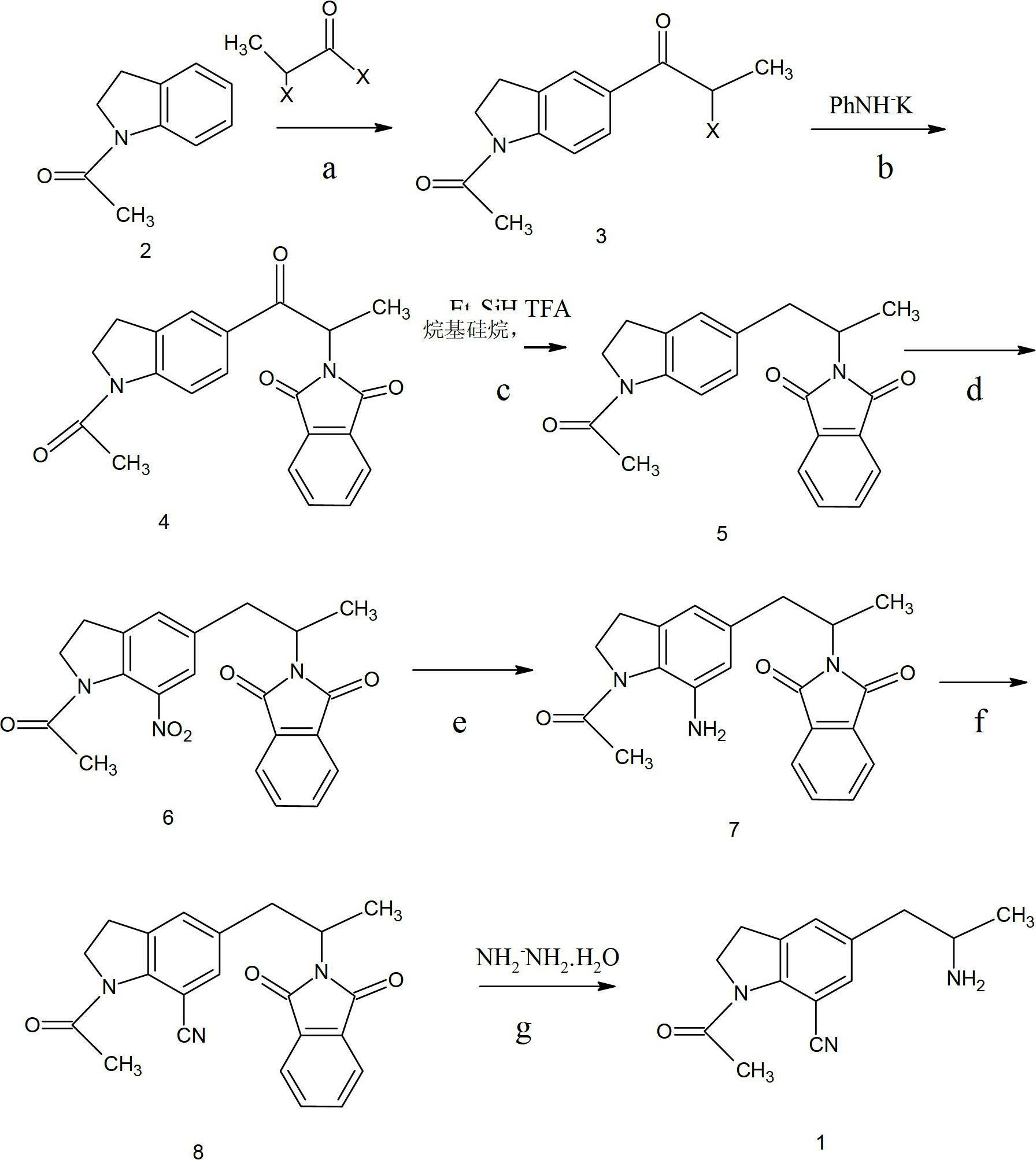
[0032] Example 1: Put 32.2g of 1-acetylindoline (that is, compound (2)), 94.5g of anhydrous aluminum trichloride and 160ml of dichloroethane into a flask, stir at room temperature, and add 33g of 2-chloropropionyl chloride dropwise .
[0033] After the dropwise addition was completed, the reaction was incubated for 8 hours, and then the reaction mixture was poured into ice water and stirred for 30 minutes.
[0034] The aqueous layer was extracted with dichloroethane, and the organic phases were combined, washed with water, then with saturated brine, and dried over sodium sulfate.
[0035] The solvent was distilled off under reduced pressure, ethanol was added to the residue, and 40.3 g of compound (3) was obtained by recrystallization with a yield of 80%.
example 2
[0036] Example 2: Put 32.2g of 1-acetylindoline (ie compound (2)), 100g of anhydrous zinc chloride and 160ml of dichloromethane into a flask, stir at room temperature, and add 33g of 2-chloropropionyl chloride dropwise.
[0037] After the dropwise addition was completed, the reaction was carried out at 50° C. for 10 hours, and then the reaction mixture was poured into ice water and stirred for 30 minutes.
[0038] The aqueous layer was extracted with dichloromethane, and the organic phases were combined, washed with water, then with saturated brine, and dried over sodium sulfate.
[0039] The solvent was distilled off under reduced pressure, ethanol was added to the residue, and 38 g of compound (3) was obtained by recrystallization with a yield of 78%.
[0040] b: Preparation of 1-acetyl-5-(2-phthalimidopropionyl), compound (4):
[0041] 50 g of compound (3), 48 g of phthalimide potassium salt, and 400 ml of DMF were put into a flask, stirred and heated to 80-100° C., and re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com