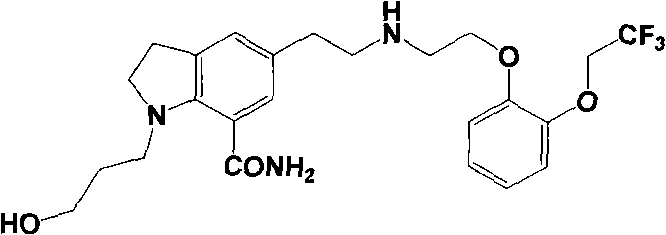
Preparation method of beta type silodosin crystal
A technology of silodosin and beta crystal form, which is applied in the field of medicinal chemistry, can solve the problems of many process steps, low yield and purity of beta crystal form silodosin, and is suitable for large-scale industrial production. High purity, high yield, simple and convenient process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of silodosin in β crystal form
[0028] 1) Weigh 1.0g of crude silodosin into a round bottom flask, add 5ml of chloroform and stir to dissolve, then add 10ml of cyclohexane and stir evenly, filter, crystallize the filtrate at 0-5°C for 0-2 hours, filter The crystals were collected and dried to obtain 0.8 g of silodosin in the β crystal form, with a purity of 99.43%, a melting point of 104.3-106.1°C, and a yield of 80.0%.
[0029] 2) Weigh 1.0g of crude silodosin into a round bottom flask, add 5ml of chloroform and stir to dissolve, then add 20ml of ether and stir evenly, filter, crystallize the filtrate at 0-5°C for 0-2 hours, and collect the crystals by filtration , and dried to obtain 0.8 g of silodosin in the β crystal form, with a purity of 99.50%, a melting point of 104.5-105.7°C, and a yield of 80.0%.
[0030] 3) Weigh 1.0g of crude silodosin into a round bottom flask, add 5ml of chloroform and stir to dissolve, then add 15ml of isopropy...
Embodiment 2
[0039] Embodiment 2: Determination of silodosin in β crystal form
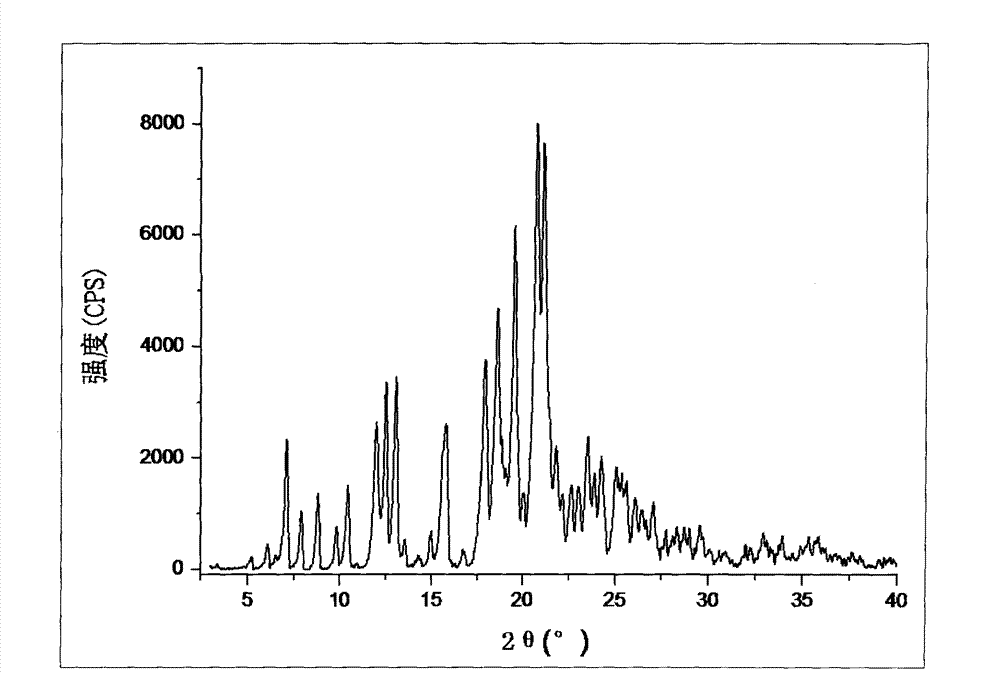
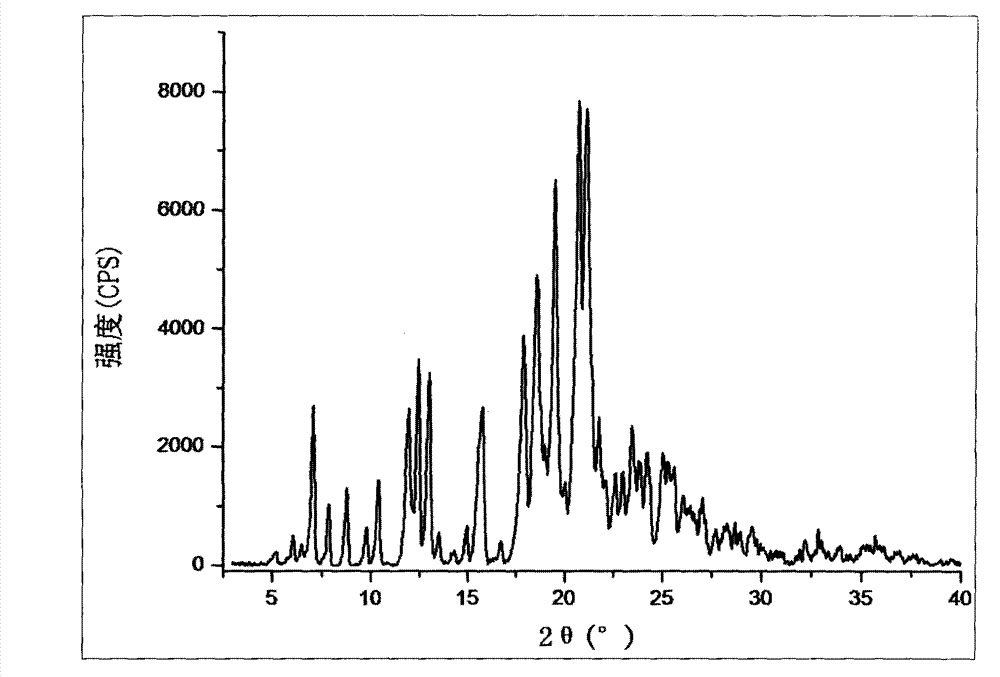
[0040] Operation steps: Carry out the X-ray diffraction (XRD) pattern determination of the β crystal form silodosin prepared by 1)-11) in Example 1, wherein the Cu-Ka radiation condition The Bragg 2θ angle is 3-40°.
[0041] The β crystal form silodosin XRD figure prepared by 1) and 5) method in embodiment 1 is respectively as follows figure 1 , figure 2 shown.
[0042] Table 1 shows the X-ray diffraction data of the β-crystal form silodosin obtained from 8) and 10) in Example 1.
[0043] Table 1 Example 1 8) and 10) obtained β crystal form silodosin X-ray diffraction data
[0044]
Embodiment 3
[0045] Embodiment 3: Oral formulation of silodosin in β crystal form
[0046] The β-crystal form silodosin prepared from 1)-11) in Example 1 was prepared into an oral dosage form.
[0047] Capsule 1
[0048] formula:
[0049]
[0050] According to the above formula, 1000 capsules containing 2.0 mg of silodosin in β crystal form were prepared by conventional methods.
[0051] Capsule 1
[0052] formula:
[0053]
[0054] According to the above formula, 1000 capsules containing 2.0 mg of silodosin in β crystal form were prepared by conventional methods.
[0055] piece 1
[0056]
[0057] According to the above formula, 1000 tablets of β-crystal form silodosin tablets containing 4.0 mg were prepared by conventional methods.
[0058] piece 2
[0059]
[0060] According to the above formula, 1000 tablets of β-crystal form silodosin tablets containing 4.0 mg were prepared by conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com