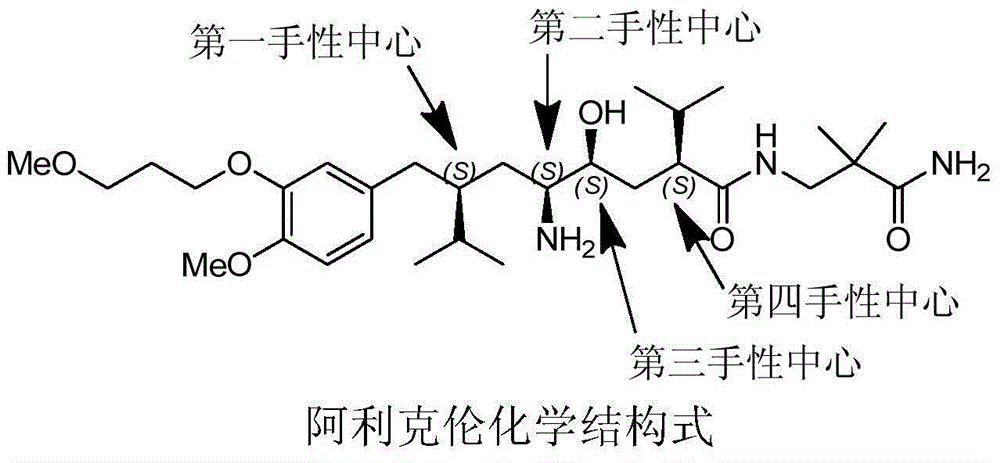
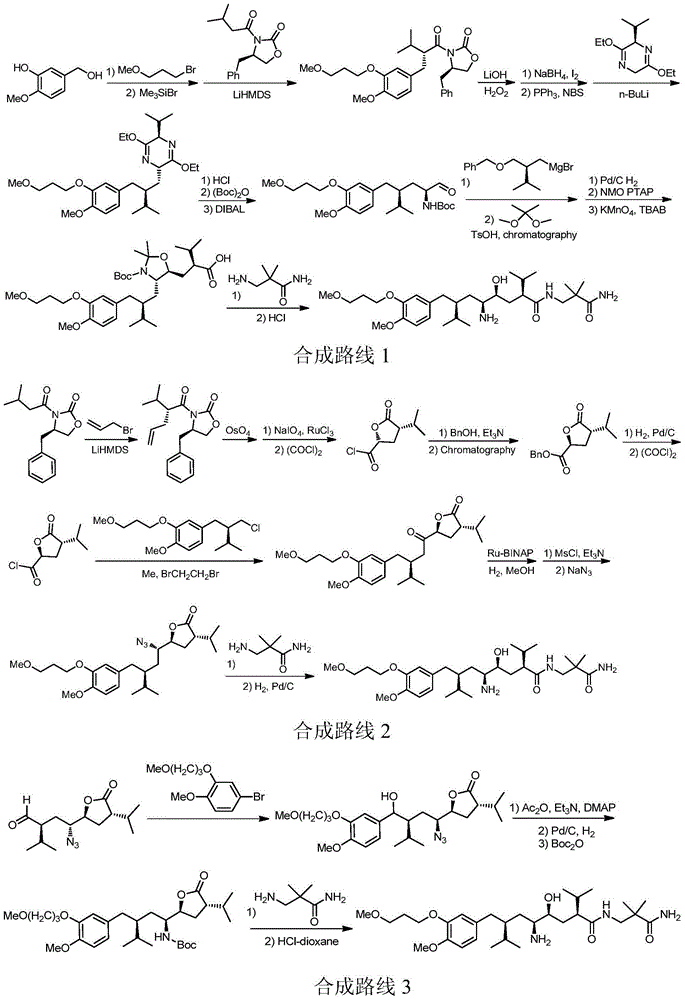
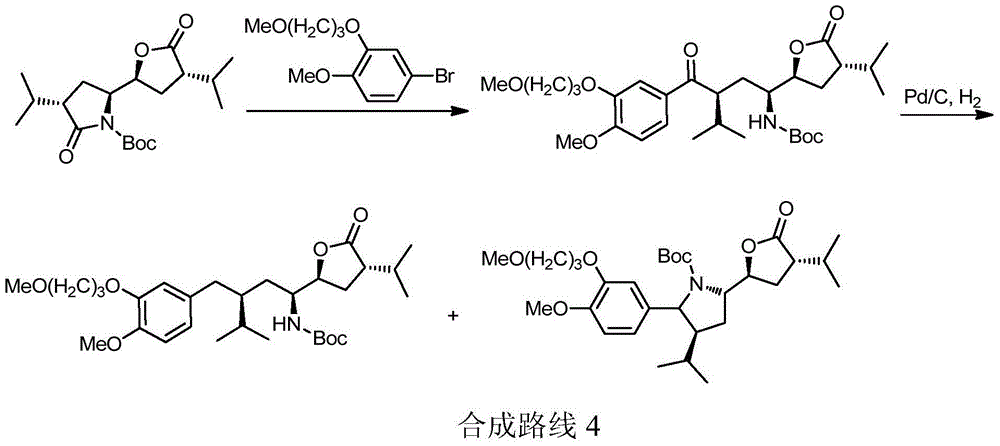
Preparation method of aliskiren
A compound and solvent technology, applied in the field of aliskiren preparation, can solve the problems of low equipment utilization, complicated operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0156] The raw materials and equipment used in the specific embodiment of the present invention are known products obtained by purchasing commercially available products.
[0157] The meaning of the acronym:
[0158] THF: Tetrahydrofuran;
[0159] MTBE: methyl tert-butyl ether;
[0160] TLC: thin layer chromatography;
[0161] DMSO: dimethyl sulfoxide;
[0162] DCM: dichloromethane;
[0163] BF 3 -Et 2 O: boron trifluoride ether solution;
[0164] TMSCN: Trimethylcyanosilane;
[0165] NMMO: N-methylmorpholine-N-oxide;
[0166] TPAP: tetra-n-propylammonium perruthenate.
[0167] Embodiment The preparation method of Aliskiren of the present invention
[0168] Preparation of (R)-3-((S)-2-isopropyl-4-valeroyl)-4-benzyloxazolidin-2-one (compound Ⅱ)
[0169] Add diisopropylamine (60.7g, 0.6mol) and THF (400mL) into a 3L three-necked flask, cool down to -78°C, and slowly add 0.6mol n-butyllithium (240mL, 2.5mol / L n-hexane solution) dropwise under nitrogen protection ), th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com