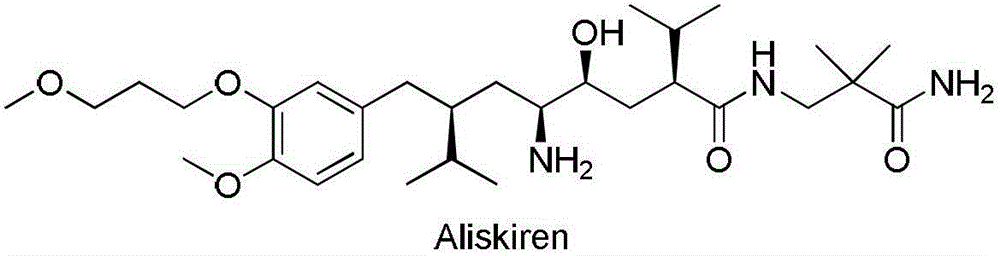
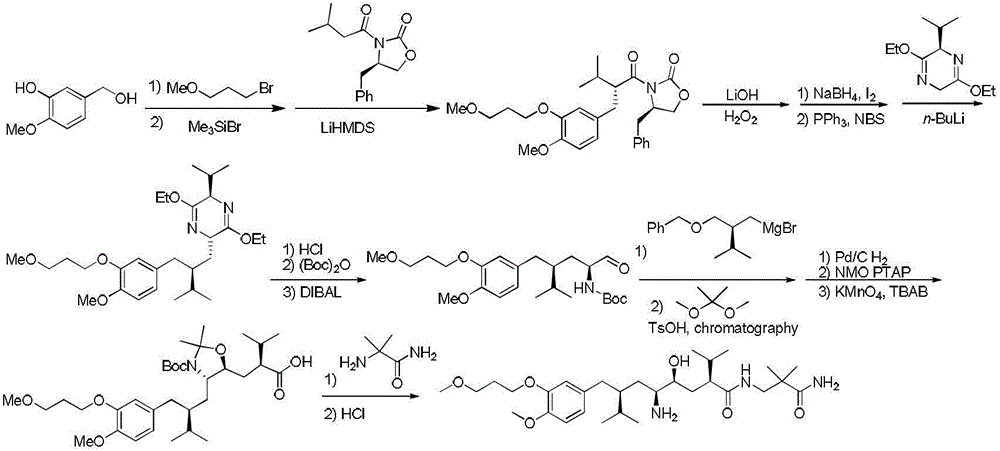
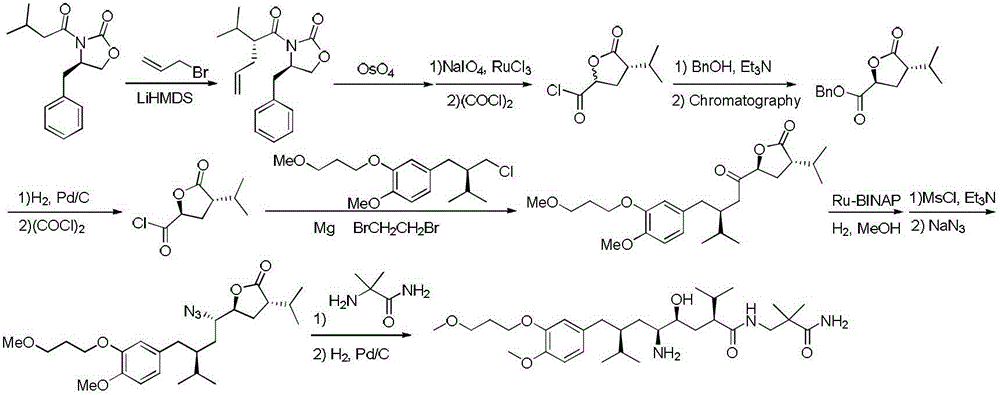
Preparation method of Aliskiren
A compound and selected technology, applied in the field of medicine and chemical industry, can solve the problems of complicated operation, high cost of raw materials, low conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] {(1S,3S)-3-[4-methoxy-3-(3-methoxypropoxy)benzoyl]-1-[(2S,4S)-4-isopropyl-5- Preparation of tert-butyl oxytetrahydrofuran-2-yl]-4-methylpentyl}carbamate (Compound II)
[0079] Add 80g of 4-methoxy-3-(3-methoxypropoxy)-bromobenzene and 0.6L THF into a 2L single-necked flask, stir and cool down to -60°C, and add 120mL of 2.5M n-butyllithium dropwise. After the dropwise addition, (3S,5S)-1-tert-butoxycarbonyl-3-isopropyl-5-((2S,4S)-4-isopropyl-5-oxo- Tetrahydro-furan-2-yl)-pyrrolidin-2-one (compound I) (90 g dissolved in 0.4 L THF), the dropwise addition was completed in 40 min. Stir the reaction at -50°C to -60°C for 1 hour, spot the plate, and monitor the end point of the reaction by TLC. After the reaction was completed, 240 mL of 2N hydrochloric acid was added to quench the reaction at low temperature, and then gradually warmed to room temperature.
[0080] The resulting reaction solution was concentrated to obtain a yellow viscous liquid. 600 mL of water and 1500 ...
Embodiment 2
[0092] Preparation of compound II
[0093] Add 80g of 4-methoxy-3-(3-methoxypropoxy)-bromobenzene and 0.6LTHF into a 2L single-necked flask, stir and cool down to -70℃~-80℃, add dropwise 120mL of 2.5M n-butyl base lithium. After the dropwise addition, (3S,5S)-1-tert-butoxycarbonyl-3-isopropyl-5-((2S,4S)-4-isopropyl-5-oxo- Tetrahydro-furan-2-yl)-pyrrolidin-2-one (90g dissolved in 0.4L THF), the dropwise addition was completed in 40min. Stir the reaction at -70~-80°C for 1 hour, spot the plate, and monitor the end point of the reaction by TLC. After the reaction was completed, 200 mL of 2N hydrochloric acid was added to quench the reaction at low temperature, and then gradually warmed to room temperature.
[0094] The resulting reaction solution was concentrated to obtain a yellow viscous liquid. 600 mL of water and 1500 mL of ethyl acetate were added thereto and stirred, the layers were separated, and the organic phase was concentrated to obtain viscous crude compound II. ...
Embodiment 3
[0104] Preparation of compound II
[0105] Add 80g of 4-methoxy-3-(3-methoxypropoxy)-bromobenzene, 0.6LTH F into a 2L single-necked flask, stir and cool down to -10~-20℃, add 1M isopropyl bromide dropwise Magnesium chloride 300mL. After the dropwise addition, (3S,5S)-1-tert-butoxycarbonyl-3-isopropyl-5-((2S,4S)-4-isopropyl-5-oxo- Tetrahydro-furan-2-yl)-pyrrolidin-2-one (90g dissolved in 0.4L THF), the dropwise addition was completed in 40min. Stir the reaction at -10 to -20°C for 1 hour, spot the plate, and monitor the end point of the reaction by TLC. After the reaction was completed, 200 mL of 2N hydrochloric acid was added to quench the reaction at low temperature, and then gradually warmed to room temperature.
[0106] The resulting reaction solution was concentrated to obtain a yellow viscous liquid. 600 mL of water and 1500 mL of ethyl acetate were added thereto and stirred, the layers were separated, and the organic phase was concentrated to obtain viscous crude com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com