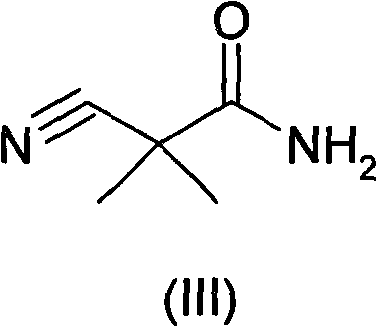
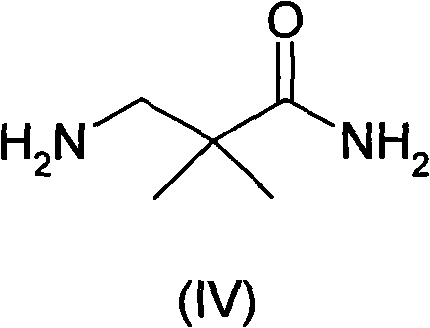
Preparation method of Aliskiren intermediate 3-amino-2,2-dimethylpropionamide
A technology of dimethylpropionamide and amino, which is applied in the field of preparation of aliskiren intermediate 3-amino-2,2-dimethylpropionamide, which can solve the difficulty of synthesis reaction that is not suitable for industrial production, has no industrial value, and Large and other problems, to achieve the effect of easy response, less loss, and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 396g of sodium methoxide solution into 500ml of anhydrous methanol, stir for half an hour after adding, add 99g of methyl cyanoacetate, heat to 65°C and reflux for 2 hours, then cool to 20-30°C, add 290g of dimethyl sulfate, add After completion, the reaction was stirred overnight at 20-30°C. Evaporate the solvent to dryness, add 500ml of water, and then extract three times with 200ml of ethyl acetate, combine the ethyl acetate layers, dry over anhydrous sodium sulfate, and evaporate the solvent to obtain 131.1g of yellow oil, namely 2,2-dimethylcyanoacetic acid Methyl ester, yield 93%.
Embodiment 2
[0025] Add 748g of sodium ethoxide solution into 500ml of absolute ethanol, stir for half an hour after adding, add 113g of ethyl cyanoacetate, heat to 80°C and reflux for 2 hours, then cool to 20-30°C, add 290g of dimethyl sulfate, add After completion, the reaction was stirred overnight at 20-30°C. Evaporate the solvent to dryness, add 500ml of water, then extract three times with 200ml of ethyl acetate, combine the ethyl acetate layers, dry over anhydrous sodium sulfate, and evaporate the solvent to obtain 134.1g of yellow oily substance, namely 2,2-dimethylcyanoacetic acid Ethyl ester, yield 95%.
Embodiment 3
[0027] Add 88g of sodium hydride into 500ml of tetrahydrofuran, stir for half an hour after adding, add 113g of ethyl cyanoacetate, stir at room temperature for 2h, add 290g of dimethyl sulfate, and stir at room temperature overnight after adding. Add 500ml of acetic acid solution, then extract three times with 200ml of ethyl acetate, combine the ethyl acetate layers, dry over anhydrous sodium sulfate, and evaporate the solvent to obtain 121.4g of yellow oil, namely ethyl 2,2-dimethylcyanoacetate , yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com