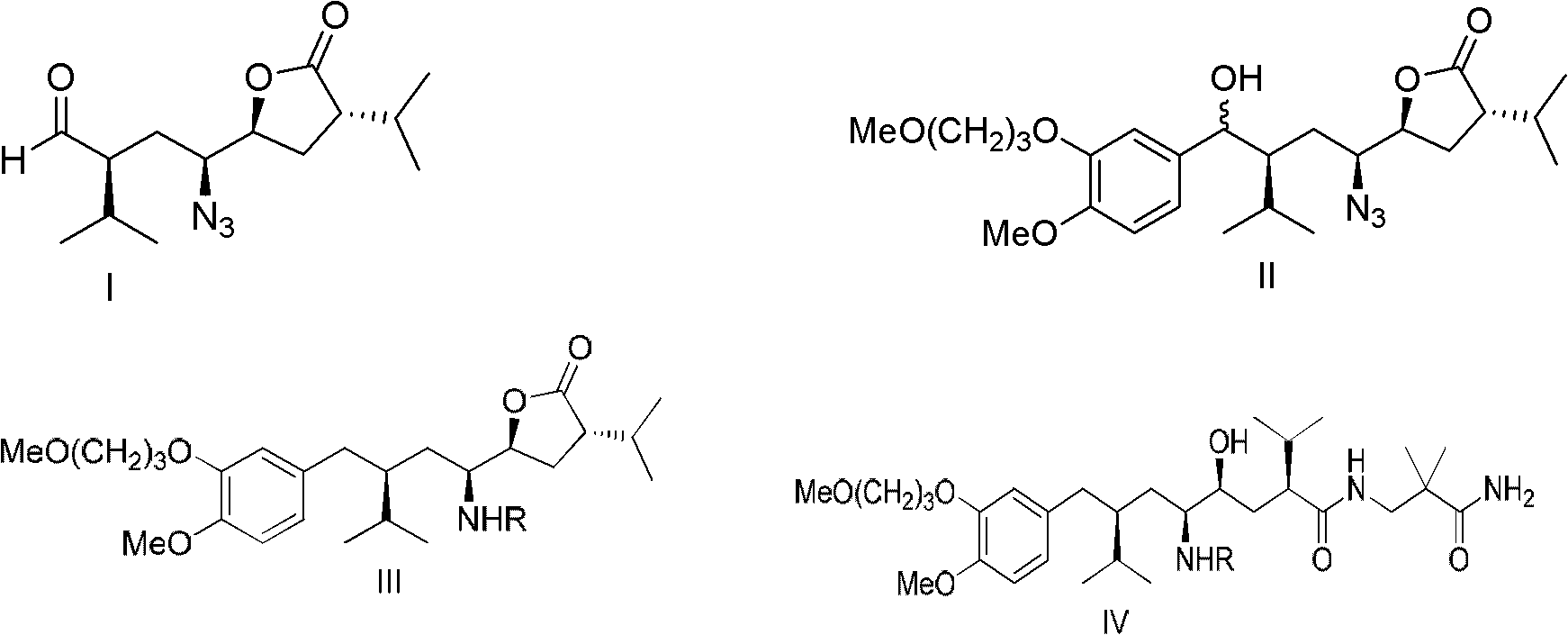
Preparation method for aliskiren
A compound and reaction time technology, applied in the field of preparation of aliskiren, can solve the problems of unsuitability for commercial production, low coupling yield, and difficult preparation of Grignard reagents, and achieve good industrial application value, short reaction route, The effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
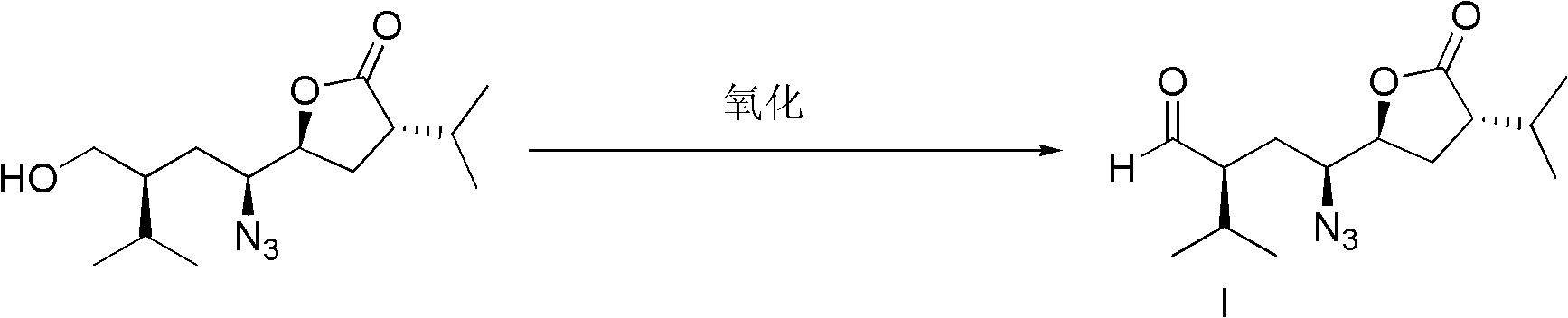
[0064] Preparation of compound (I)
[0065] 22.64g (80mmol) of (3S, 5S, 1'S, 3'S)-5-(1'-azido-3'-hydroxymethyl-4'-methyl-pentyl)-3-isopropyl- Dihydrofuran-2-one was dissolved in 80ml of dichloromethane, 125mg (0.8mmol) of 2,2,6,6-tetramethylpiperidine oxide was added thereto, the temperature was lowered to below 0°C, and 71mL (96mmol) of ) molar concentration of 1.35mol / L sodium hypochlorite aqueous solution at 50 ° C for 2 hours. The reaction solution was cooled, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 20.68 g of compound (I) with a yield of 92%.
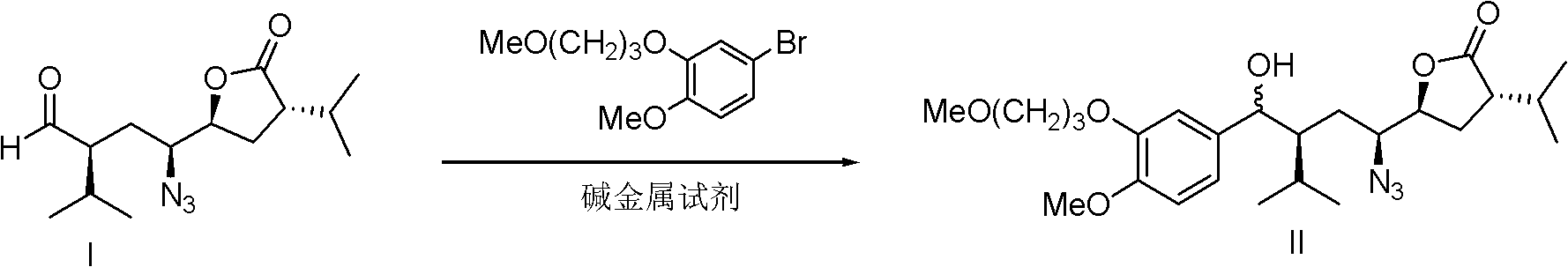
[0066] Preparation of compound (II)
[0067] Compound (I): 4-methoxy-3-(3-methoxypropoxy)-bromobenzene: metal reagent = 1: 1.4: 1.6 according to the molar ratio of the feed is carried out as follows, wherein the metal reagent is n-butyl Base Lithium:
[0068] Dissolve 3.85g (14mmol) of 4-methoxy-3-(3-methoxypropoxy)-bromobenzene in 14ml tetrahydrofuran, and add 1.6...
Embodiment 2
[0079] Preparation of compound (I)
[0080]11.3g (40mmol) of (3S, 5S, 1'S, 3'S)-5-(1'-azido-3'-hydroxymethyl-4'-methyl-pentyl)-3-isopropyl- Dihydrofuran-2-one was dissolved in 80ml of dichloromethane, 65mg (0.4mmol) of 2,2,6,6-tetramethylpiperidine oxide was added to it, the temperature was lowered to below 0°C, and 36mL (48mmol) of The molar concentration of (consumption) is 1.35mol / L sodium hypochlorite aqueous solution, react at 50 ℃ for 3 hours. The reaction solution was cooled, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 10 g of compound (I) with a yield of 89%.
[0081] Preparation of compound (II)
[0082] Compound (I): 4-methoxy-3-(3-methoxypropoxy)-bromobenzene: metal reagent = 1: 1.2: 1.4 according to the molar ratio of the feed is carried out as follows, wherein the metal reagent is n-butyl Base Lithium:
[0083] Dissolve 3.3g (12mmol) of 4-methoxy-3-(3-methoxypropoxy)-bromobenzene in 12ml of tetrahydro...
Embodiment 3
[0094] Preparation of compound (I)
[0095] Dissolve 3.1mL (35.9mmol) oxalyl chloride and 5.1mL (71.9mmol) dimethyl sulfoxide DMSO in dichloromethane, cool down to -65°C, add 6.2g (21.8mmol) (3S, 5S, 1'S, 3'S) -5-(1'-azido-3'-hydroxymethyl-4'-methyl-pentyl)-3-isopropyl-dihydrofuran-2-one in 22ml dichloromethane solution in- React at 65℃~-55℃ for 20 hours, add 3mL (21.8mmol) triethylamine, neutralize the reaction solution to neutrality, extract with ethyl acetate, dry over anhydrous sodium sulfate, concentrate to obtain light yellow liquid compound (I) 4.9g, yield 80%.
[0096] Preparation of compound (II)
[0097] Compound (I): 4-methoxy-3-(3-methoxypropoxy)-bromobenzene: metal reagent = 1: 1.4: 2.0 according to the molar ratio of the feed to carry out the following operations, wherein the metal reagent is isopropyl Magnesium Chloride:
[0098] Dissolve 3.85g (14mmol) of 4-methoxy-3-(3-methoxypropoxy)-bromobenzene in 14ml of tetrahydrofuran, and add 1.0mol / L of isopropyl t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com