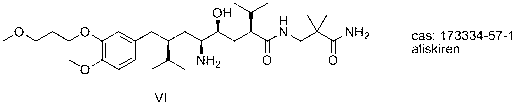
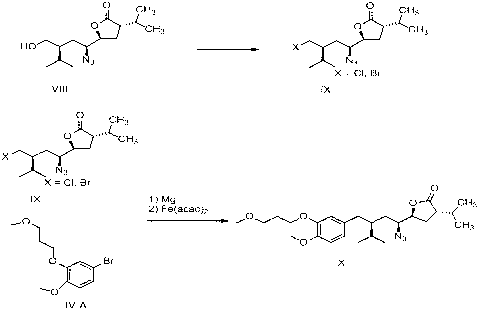
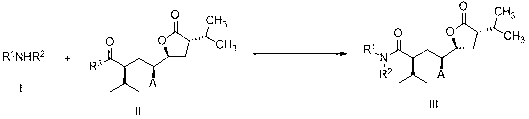Aliskiren intermediate, and preparation method and application thereof
An intermediate and reaction technology, applied in the field of organic pharmaceuticals, can solve the problems of many by-products, high cost, and long reaction time, and achieve the effects of easy preparation, high safety, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]First, weigh 13.0 g (43.7 mmol) of the compound of formula (II-a), dissolve it in 100 ml of dichloromethane, add 9.2 g (56.8 mmol) of carbonyldiimidazole, stir at room temperature for 1 hour, and gradually add the compound of formula (I-A) 5.1 g (52.4 mmol), stirred overnight, the reaction equation is shown in Reaction Equation 6:
[0044] Reaction 6
[0045] ;
[0046] The reaction solution was washed with 1 mol / L NaOH solution, left to separate the liquids, the organic phase was taken, the water phase was extracted twice with dichloromethane, the dichloromethane extract was combined with the organic phase, and the solution was washed with anhydrous sodium sulfate It was dried, filtered to remove anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude compound of formula (III-Aa), which was purified by silica gel column chromatography to obtain 13.4 g of compound (III-Aa) with a purity of 98% and a yield of 90%.
Embodiment 2
[0048] First, weigh 13.0 g (43.7 mmol) of the compound of formula (II-a), dissolve it in 100 ml of dichloromethane, add 9.2 g (56.8 mmol) of carbonyldiimidazole, stir at room temperature for 1 hour, and gradually add the compound of formula (I-B) 7.3 g (52.4 mmol), stirred overnight, the reaction equation is shown in Reaction Equation 7:
[0049] Reaction 7
[0050]
[0051] The reaction solution was washed with 1 mol / L NaOH solution, left to separate the liquids, the organic phase was taken, the water phase was extracted twice with dichloromethane, the dichloromethane extract was combined with the organic phase, and the solution was washed with anhydrous sodium sulfate Drying, filtration to remove anhydrous sodium sulfate, concentration under reduced pressure to obtain the crude compound of formula (III-Ba), which was purified by silica gel column chromatography to obtain 14.4 g of compound (III-Aa) with a purity of 98% and a yield of 86%.
[0052]
Embodiment 3
[0054] First, weigh 7.8 g (26.2 mmol) of the compound of formula (II-a), dissolve it in 60 ml of dichloromethane, add 5.5 g (33.9 mmol) of carbonyldiimidazole, stir at room temperature for 1 hour, and gradually add the compound of formula (I-C) 6.3 g (31.4 mmol), stirred overnight, the reaction equation is shown in Reaction Equation 8:
[0055] Reaction 8
[0056]
[0057] The reaction solution was washed with 1 mol / L NaOH solution, left to separate the liquids, the organic phase was taken, the water phase was extracted twice with dichloromethane, the dichloromethane extract was combined with the organic phase, and the solution was washed with anhydrous sodium sulfate It was dried, filtered to remove anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude compound of formula (III-Ca), which was purified by silica gel column chromatography to obtain 9.7 g of (III-Ca) compound with a purity of 97% and a yield of 84%.
[0058]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com