Practical synthesis method for feritin inhibitor aliskiren
A synthetic method and high blood pressure technology, applied in the direction of chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of shortening the steps of the reaction, racemic products, etc., and achieve shortening of the steps of the reaction, reaction Reasonable effect of process selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
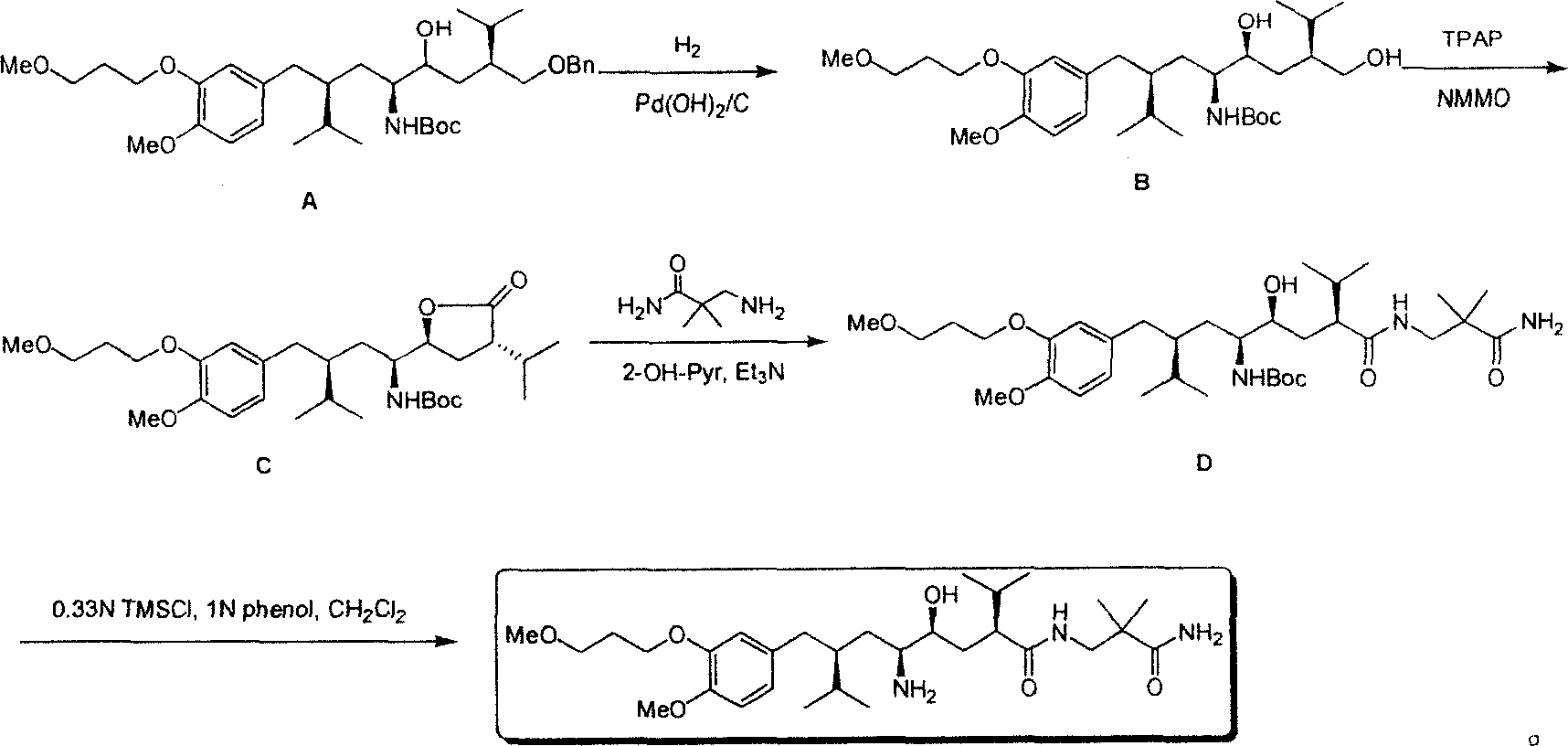
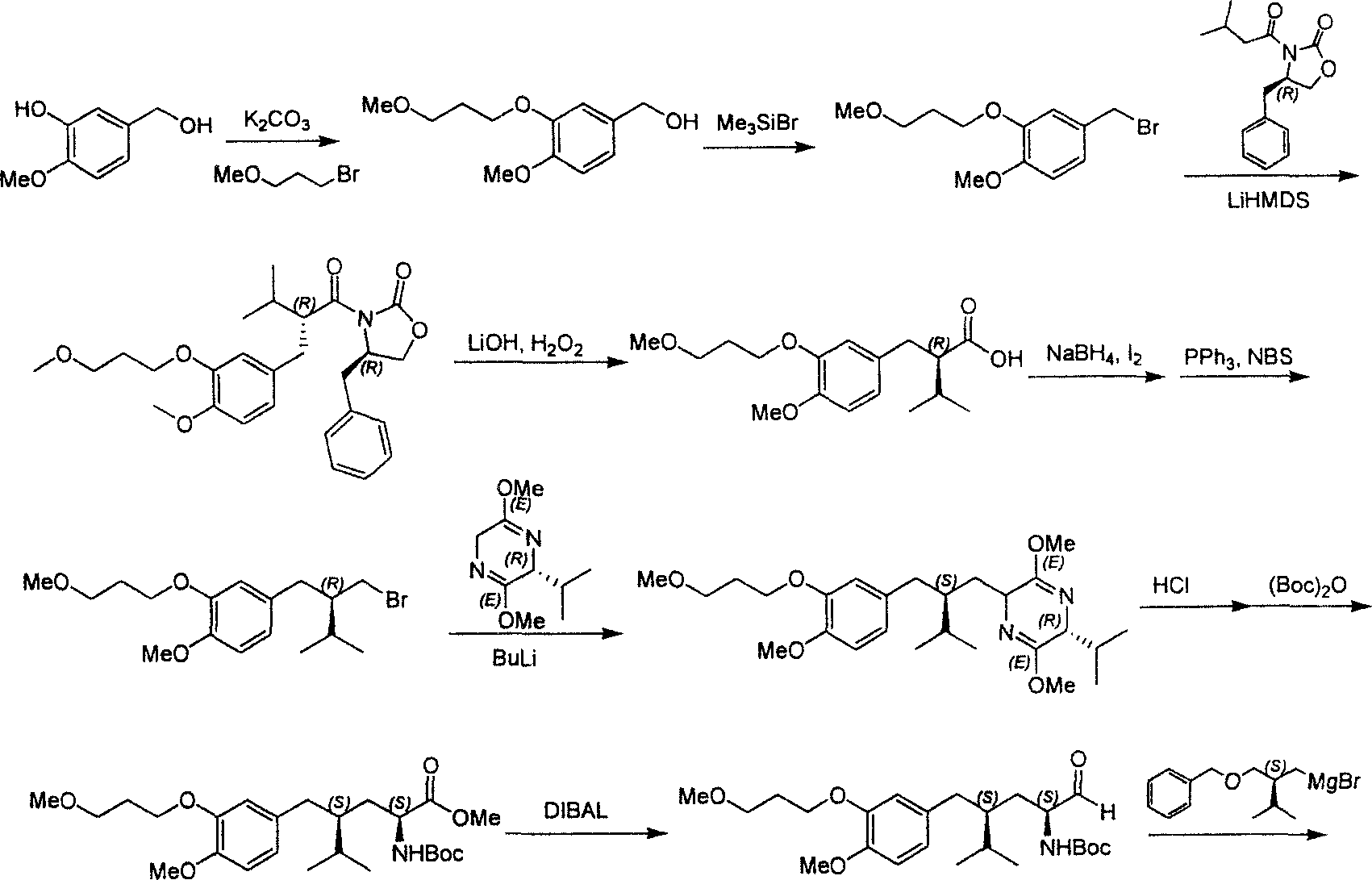
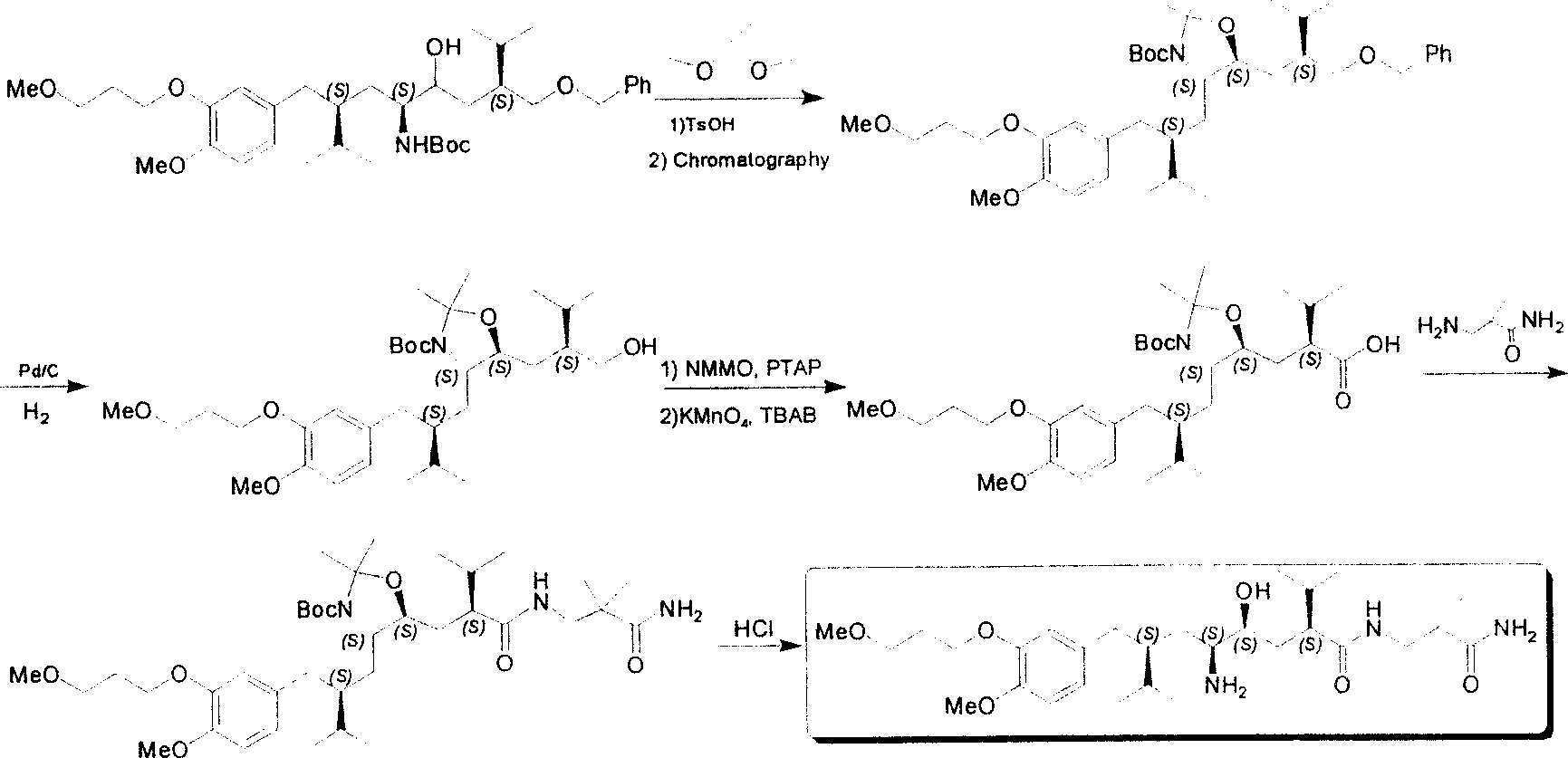
[0033] Synthesis of Aliskiren:
[0034] The second step: (1S, 2S, 4S, 2’S)-(2-hydroxyl-4-hydroxymethyl-1-{2-[4-methoxyl-3-(3-methoxyl) Synthesis of -propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-tert-butyl carbonate
[0035] The method described in document 1 first prepares intermediate A, then intermediate A (16g, 25.4mmol) is dissolved in the absolute methanol of 200ml, adds the Pd / C catalyst (0.8g) of 5%, in 1 Atmospheric pressure, hydrogenation at 40°C for 12 hours. The catalyst was removed by filtration, spin-dried, and column chromatography (PE: EA = 3: 1) gave intermediate B (6 g, 17.8 mmol) and its diastereomer (3.8 g, 7.1 mmol), yield 98% . 1 HNMR (300ppm, CDCl3): δ6.78-6.68 (m, 3H), 4.71 (brs, 1H), 4.12-4.07 (t, J=6.3Hz, 2H), 3.82 (s, 3H), 3.72-3.44 ( m, 6H), 3.35(s, 3H), 2.64(brs, 2H), 2.50(m, 2H), 2.10(m, 2H), 1.72-1.45(m, 8H), 1.41(s, 9H), 0.90 (m, 12H); MS (m / z): 540 (M+H) + .
[0036] The second step: (1S, 3S, 1'S, 4'S)-{1-(4-isopropyl-5-oxo-tetr...
Embodiment 2
[0043] Synthesis of Aliskiren:
[0044] The first step: (1S, 2S, 4S, 2’S)-(2-hydroxy-4-hydroxymethyl-1-{2-[4-methoxy-3-(3-methoxy Synthesis of -propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-tert-butyl carbonate
[0045] The method described in document 1 first prepares intermediate A, then intermediate A (21g, 33.4mmol) is dissolved in the anhydrous methanol of 300ml, adds Raney Ni catalyst (2.1g), at 10 atmospheric pressure, 70 hydrogenation at °C for 12 hours. The catalyst was removed by filtration, spin-dried, and column chromatography (PE:EA=3:1) gave Intermediate B (11.4g, 21.2mmol) and its diastereomer (4.6g, 8.5mmol), yield 89 %. Its test data is as shown in the first step of the above-mentioned embodiment 1.
[0046] The second step: (1S, 3S, 1'S, 4'S)-{1-(4-isopropyl-5-oxo-tetrahydrofuryl-2-yl)-3-[4- Synthesis of methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-tert-butyl carbonate
[0047] According to the process conditions and operation steps ...
Embodiment 3
[0053] Synthesis of Aliskiren:
[0054] The first step: (1S, 4S, 2'S)-(2-hydroxy-4-hydroxymethyl-1-{2-[4-methoxy-3-(3-methoxy Synthesis of -propoxy)-benzyl]-3-methyl-butyl}-5-methyl-hexyl)-tert-butyl carbonate
[0055] The method described in document 1 first prepares intermediate A, then intermediate A (30g, 47.7mmol) is dissolved in the dehydrated alcohol of 400ml, adds the Pd / C catalyst (1.5g) of 5%, in 3 Atmospheric pressure, hydrogenation at room temperature for 12 hours. The catalyst was removed by filtration, spin-dried, and column chromatography (PE: EA = 3: 1) gave intermediate B (17.3 g, 32.1 mmol) and its diastereomer (7.1 g, 13.2 mmol), yield 95 %. Its test data is as shown in the first step of the above-mentioned embodiment 1.
[0056] The second step: (1S, 3S, 1'S, 4'S)-{1-(4-isopropyl-5-oxo-tetrahydrofuryl-2 base)-3-[4- Synthesis of methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-tert-butyl carbonate
[0057] According to the process conditio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com