Method for synthesizing substituted amino carboxylic acid by ugi reaction
A technology of aminocarboxylic acid and Uji reaction, which is applied in the field of synthesizing substituted aminocarboxylic acids by Uji reaction, which can solve the problems of harsh reaction conditions and low industrialization level, and achieve the effect of reasonable selection of reaction process and avoiding low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
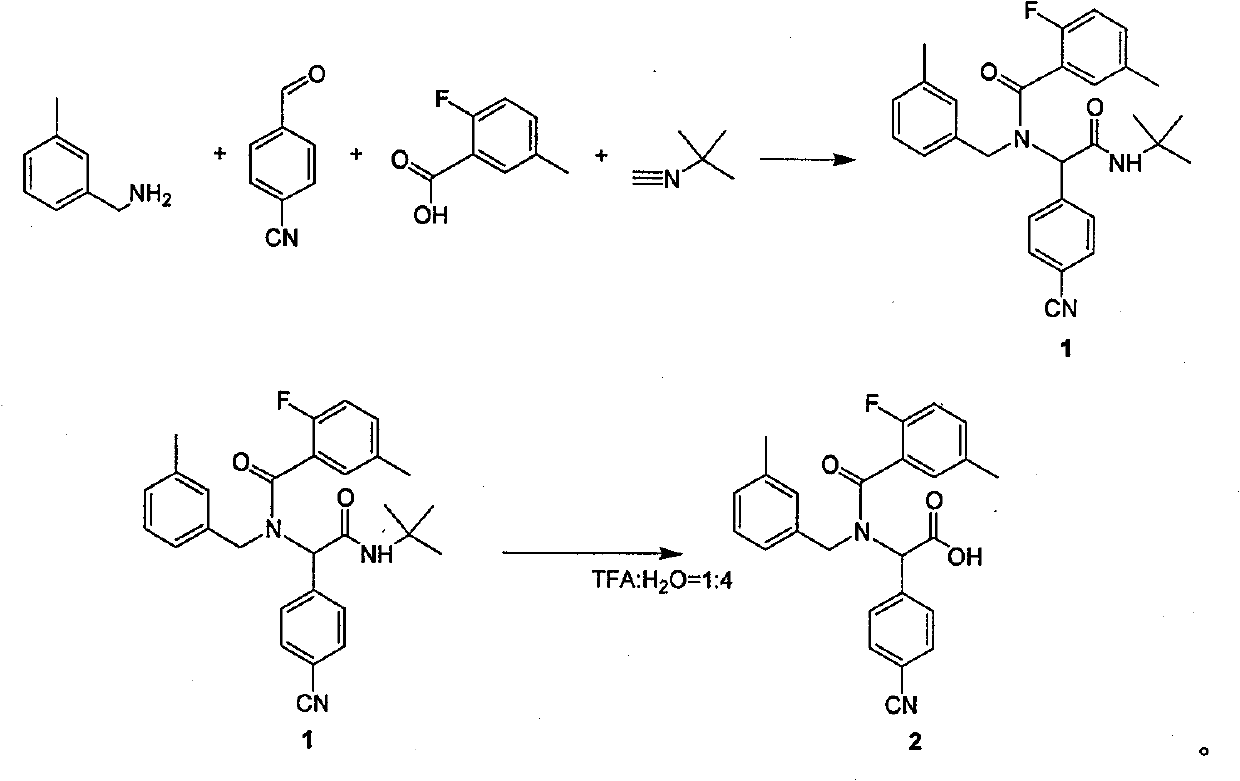
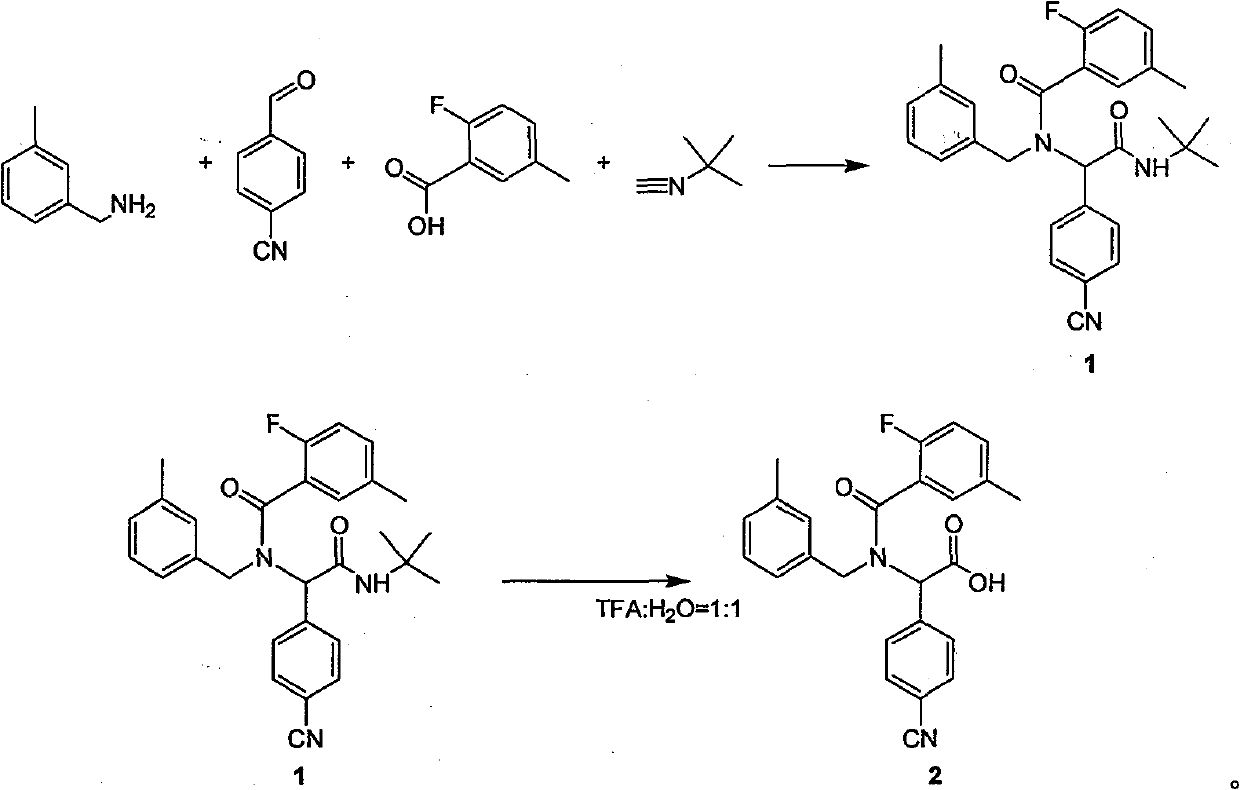
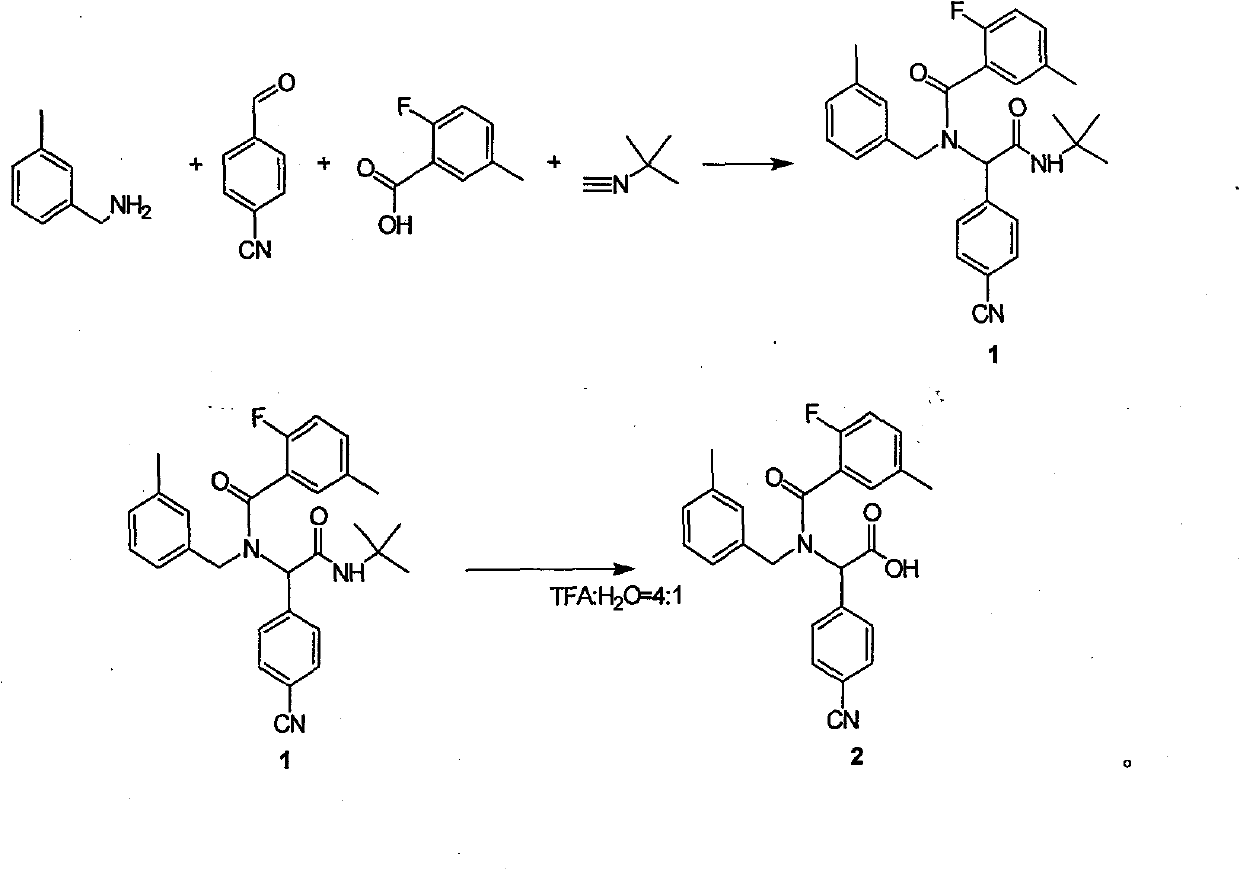
[0011] 1 Preparation of dipeptide protected by tert-butyl group:
[0012] Add anhydrous methanol (100ml) to a dry 100ml round-bottomed flask, then add 4-cyanobenzaldehyde (1g, 7.63mmol), replace nitrogen in the reaction flask three times, and slowly add 3-Methylbenzylamine (0.925 g, 7.63 mmol) kept the reaction temperature not higher than 10 °C. After the feeding is completed, the solution turns yellow. After stirring and reacting for 1 hour at 10°C, 2-fluoro-5-methylbenzoic acid (1.18g, 7.63mmol) and tert-butylisocyanate (0.641g, 7.63mmol) are sequentially added under nitrogen protection. ), keep the reaction temperature not higher than 10°C, after feeding is completed, the reaction solution is raised to room temperature and stirred for 24 hours. Until TLC (petroleum ether / ethyl acetate=5:1) detected that the starting material disappeared, the reaction was complete. The reaction solution was spin-dried under reduced pressure. The product 1 (3.32 g, 7.04 mmol) was obtained ...
Embodiment 2
[0019] 1 Preparation of dipeptide protected by tert-butyl group:
[0020] Add anhydrous methanol (100ml) to a dry 100ml round-bottomed flask, then add 4-cyanobenzaldehyde (1g, 7.63mmol), replace nitrogen in the reaction flask three times, and slowly add 3-Methylbenzylamine (0.925 g, 7.63 mmol) kept the reaction temperature not higher than 10 °C. After the feeding is completed, the solution turns yellow. After stirring and reacting for 1 hour at 10°C, 2-fluoro-5-methylbenzoic acid (1.18g, 7.63mmol) and tert-butylisocyanate (0.641g, 7.63mmol) are sequentially added under nitrogen protection. ), keep the reaction temperature not higher than 10°C, after feeding is completed, the reaction solution is raised to room temperature and stirred for 24 hours. Until TLC (petroleum ether / ethyl acetate=5:1) detected that the starting material disappeared, the reaction was complete. The reaction solution was spin-dried under reduced pressure. The product 1 (3.32 g, 7.04 mmol) was obtained ...
Embodiment 3
[0027] 1 Preparation of dipeptide protected by tert-butyl group:
[0028] Add anhydrous methanol (100ml) to a dry 100ml round-bottomed flask, then add 4-cyanobenzaldehyde (1g, 7.63mmol), replace nitrogen in the reaction flask three times, and slowly add 3-Methylbenzylamine (0.925 g, 7.63 mmol) kept the reaction temperature not higher than 10 °C. After the feeding is completed, the solution turns yellow. After stirring and reacting for 1 hour at 10°C, 2-fluoro-5-methylbenzoic acid (1.18g, 7.63mmol) and tert-butylisocyanate (0.641g, 7.63mmol) are sequentially added under nitrogen protection. ), keep the reaction temperature not higher than 10°C, after feeding is completed, the reaction solution is raised to room temperature and stirred for 24 hours. Until TLC (petroleum ether / ethyl acetate=5:1) detected that the starting material disappeared, the reaction was complete. The reaction solution was spin-dried under reduced pressure. The product 1 (3.32 g, 7.04 mmol) was obtained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com