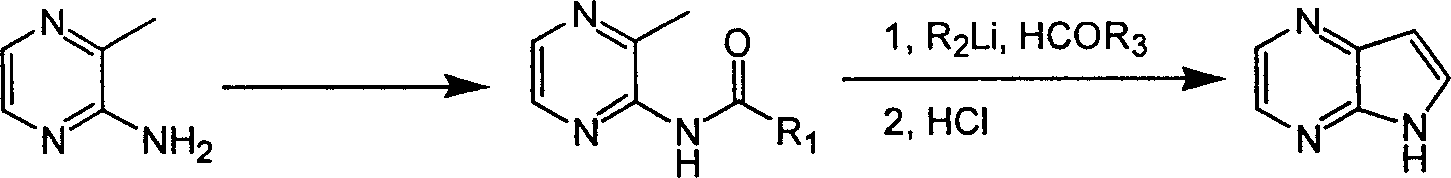
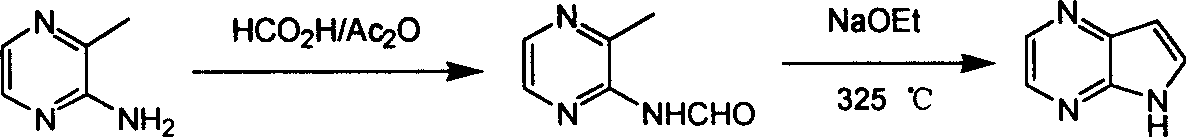
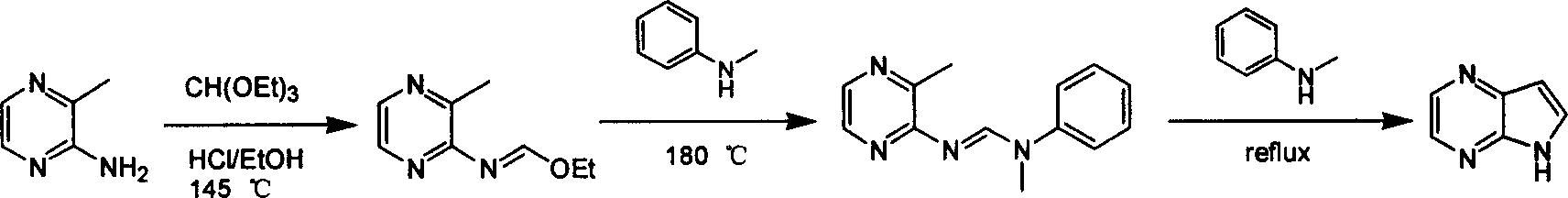
Preparing process of 4,4-diazaindole
A technology of diaza and indole, which is applied in the field of organic synthesis, can solve problems such as unfavorable experimental operations, and achieve the effect of avoiding high temperature conditions and choosing a reasonable reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. Synthesis of 4,7-diazaindole
[0019] The first step: the synthesis of 2-amino-3-methylpyrazine
[0020] Referring to the process of US5861401, it is obtained by reacting 2-chloro-3-methylpyrazine with ammonia water, and the yield is 71%. 1 H NMR (400MHz, DMSO-d 6 ): δ 7.72 (d, J = 2.4 Hz, 1H), 7.56 (d, J = 2.4, 1H), 6.10 (s, br, 2H), 2.22 (s, 3H).
[0021] The second step: the synthesis of 2-pivalamido-3-methylpyrazine
[0022] 2-Amino-3-methylpyrazine (24 g, 0.22 mol) was dissolved in dichloromethane (500 mL) and triethylamine (63 mL, 0.44 mol). Cool to 0°C under nitrogen protection, and add (40 g, 0.33 mol) pivaloyl chloride dropwise. After completion, the temperature was naturally raised to room temperature and stirred for 40 minutes, and then heated and stirred at 40° C. for 4 hours. TLC followed the reaction. After the reaction was completed, the reaction solution was poured into ice water, and the organic phase was washed with saturated sodium bicar...
Embodiment 2
[0026] 2. Synthesis of 4,7-diazaindole
[0027] Anhydrous THF (40 mL) was added into a 250 mL three-neck flask, cooled to -10°C under nitrogen protection, and n-butyllithium (2.5M n-hexane solution, 10 mL) was added dropwise. Control the temperature -10-5°C, add dropwise anhydrous THF solution (20ml) of 2-pivalamido-3-methylpyrazine (1.93g, 10mmol) after stirring for 10 minutes, control the temperature during the process -5-0 ℃. After stirring at this temperature for 4 hours, N-formylpiperidine (0.36 g, 3.2 mmol) was added dropwise. After stirring at 0°C for 40 minutes, the reaction solution was raised to room temperature, 3M HCl (40mL) was added, and the layers were separated after stirring for 0.5 hours. The organic phase was extracted with 3M HCl (2×15mL), and the combined aqueous phase was washed with ethyl acetate (50mL). Wash, then heat at 100°C for 6 hours. The reaction was tracked by TLC. After the reaction was completed, the pH was adjusted to 8-9 with 6M NaOH, a...
Embodiment 3
[0029] 3. Synthesis of 4,7-diazaindole
[0030] The first step: the synthesis of 2-tert-butoxycarboxamido-3-methylpyrazine
[0031] 2-Amino-3-methylpyrazine (1.09 g, 10 mmol) was dissolved in acetonitrile (40 mL) and triethylamine (2.02 g, 20 mol). At room temperature, 4-(N,N-dimethylamino)pyridine (0.12 g, 1 mmol) was added, and then a solution of Boc anhydride (3.27 g, 20 mmol) in acetonitrile (5 mL) was added dropwise, and stirred overnight at room temperature. TLC tracked the reaction, and found that the raw materials were not completely reacted, and the reaction solution was heated to reflux for 4 hours. After the reaction, the reaction solution was concentrated and chromatographically obtained 2-tert-butoxycarboxamido-3-methylpyrazine (1.17 g, 5.6 mmol, yield 56%). 1 H NMR (400MHz, CDCl 3 ): δ8.39(d, J=2.4Hz, 1H), 8.25(d, J=2.4, 1H), 7.98(s, br, 1H), 2.51(s, 3H), 1.45(s, 9H).
[0032] The second step: the synthesis of 4,7-diazaindole
[0033] Anhydrous THF (20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com