Method for preparing aliskiren key intermediate by enzyme method
An enzymatic preparation and intermediate technology, applied in the field of biomedicine, can solve the problems of large discharge of organic three wastes, unsuitable for industrial production, high pressure conditions, etc., and achieves low production cost, good industrial application value, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 2,2-dimethyl-3-aminopropionamide
[0032] Step a)
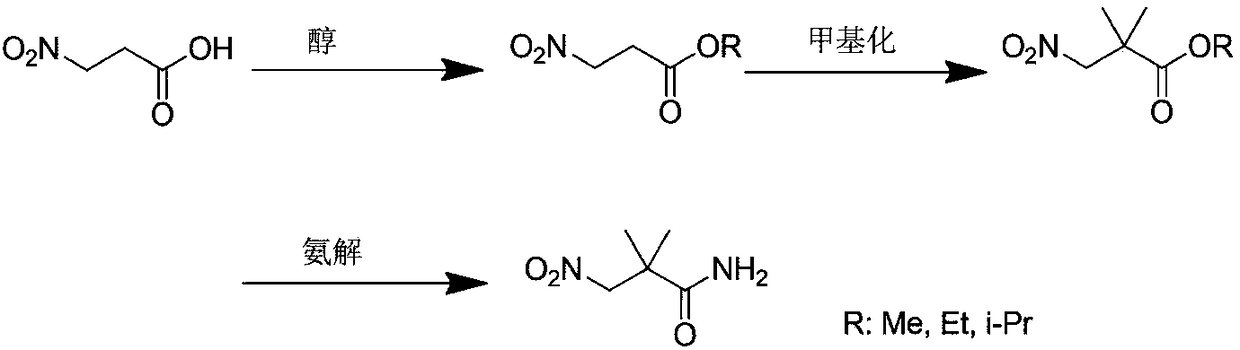
[0033] 3-Nitropropionic acid (10g) and methanol (8L) were added to the reactor, concentrated sulfuric acid (2ml) was added dropwise, reacted for 0.5h under reflux conditions, and distilled under reduced pressure to obtain 3-nitropropionic acid methyl ester (11g), Put it directly into the next step.
[0034] Step b)
[0035] Add methyl 3-nitropropionate (11g), sodium methoxide (4.8g) and methanol (8L) into the reactor, react under reflux for 2h, then cool to 20-30°C, add dimethyl sulfate (11g) , stirred overnight at room temperature. The reaction solvent was distilled under reduced pressure, water (5 L) was added, extracted with ethyl acetate (3 L*3), dried, and distilled under reduced pressure to obtain methyl 2,2-dimethyl-3-nitropropionate (11.4 g).
[0036] Step c)
[0037] 2,2-Dimethyl-3-nitropropionic acid methyl ester (11.4g) was dissolved in methanol (10L), passed through ammonia, st...
Embodiment 2
[0040] Example 2 Preparation of 2,2-dimethyl-3-aminopropanamide
[0041] Step a)
[0042] 3-Nitropropionic acid (100g) and methanol (80L) were added in the reactor, concentrated sulfuric acid (20ml) was added dropwise, reacted under reflux conditions for 0.5h, and distilled under reduced pressure to obtain 3-nitropropionic acid methyl ester (110g), Put it directly into the next step.
[0043] Step b)
[0044] Add methyl 3-nitropropionate (110g), sodium methoxide (48g), and methanol (20L) into the reactor, react for 2 hours under reflux, then cool to 20-30°C, add dimethyl sulfate (110g), Stir overnight at room temperature. The reaction solvent was distilled under reduced pressure, water (15 L) was added, extracted with ethyl acetate (10 L*3), dried, and distilled under reduced pressure to obtain methyl 2,2-dimethyl-3-nitropropionate (114 g).
[0045] Step c)
[0046] 2,2-Dimethyl-3-nitropropionic acid methyl ester (114g) was dissolved in methanol (10L), passed through ammo...
Embodiment 3
[0049] Example 3 Preparation of 2,2-dimethyl-3-aminopropionamide
[0050] Step a), b), c) are the same as in Example 1;
[0051] Step d)
[0052] 2,2-Dimethyl-3-nitropropionamide (10.3g), nitroreductase enzyme powder (5mg), NADPH (0.5mg), Tris-HCl buffer (30mM, 0.05L, pH=6) , stirred at 70°C for 12h, filtered, extracted with ethyl acetate, dried and concentrated to obtain the crude product, which was recrystallized in petroleum ether to obtain the product 2,2-dimethyl-3-aminopropionamide (7.36g, yield 90%) , the product purity was 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com