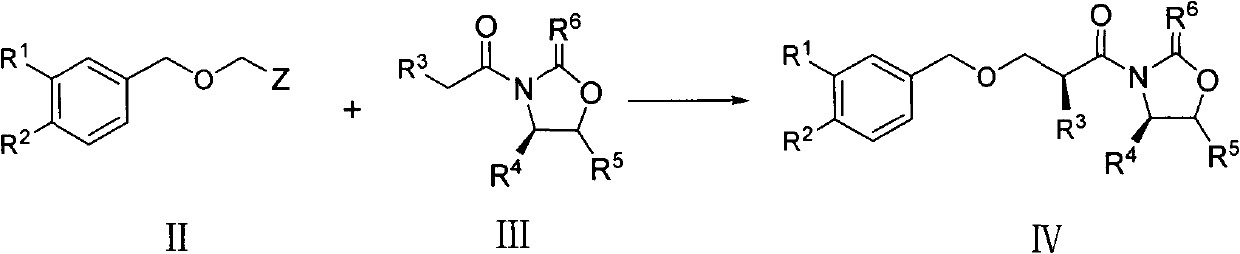
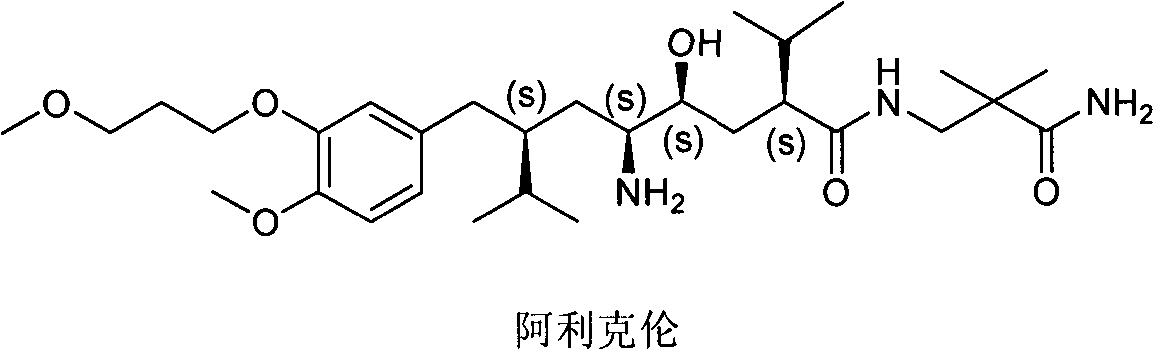
Preparation method of aliskiren intermediate
An inert gas, straight-chain technology, applied in the field of preparation of aliskiren intermediates, can solve the problems of complicated post-processing operations, unfavorable large-scale production, low reaction yield, etc., and achieves improved efficiency, improved yield, and wide application. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 compound IVa
[0042]
[0043] Under nitrogen protection, compound IIIa (40.0 g, 153.2 mmol) was dissolved in 500 ml of anhydrous CH 2 Cl 2 , cooled to 0°C, dropwise added TiCl 4 (18ml, 163.8mmol), dropwise, the solution was yellow, stirred for 5min; dropwise added N, N-diisopropylethylamine (30ml, 174mmol), dropwise, the reaction solution turned black, stirred for 1 hour; dropwise Compound II a (44ml, 316.8mmol) was reacted at 0°C for 20 hours after dropping, and the reaction solution gradually turned yellow. Add 200ml saturated NH 4 Cl aqueous solution and 320ml water, stir, separate liquid, use CH for water phase 2 Cl 2 (70ml×2) extraction, combined organic phase, washed with water and saturated brine successively, anhydrous MgSO 4 dry. Filtration and concentration under reduced pressure gave an oil (50.2 g, 85.8%), which was recrystallized from n-hexane and petroleum ether to give 46.9 g of a white solid with a yield of 80.1...
Embodiment 2
[0049] The preparation of embodiment 2 compound IVa
[0050]
[0051] Under nitrogen protection, compound IIIa (40.0g, 153.2mmol) was dissolved in 500ml anhydrous tetrahydrofuran, cooled to 0°C, and TiCl was added dropwise 4 (18ml, 163.8mmol), dropwise, the solution was yellow, stirred for 5min; dropwise added N, N-diisopropylethylamine (30ml, 174mmol), dropwise, the reaction solution turned black, stirred for 1 hour; dropwise Compound IIa (44ml, 316.8mmol) was reacted at 0°C for 20 hours after dropping, and the reaction solution gradually turned yellow. Add 200ml saturated NH 4 Cl aqueous solution and 320ml water, stirred, separated, the aqueous phase was extracted with ethyl acetate (70ml×2), the organic phases were combined, washed with water and saturated brine successively, anhydrous MgSO 4 dry. Filtrate and concentrate under reduced pressure to obtain an oil, which was recrystallized from n-hexane and petroleum ether to obtain 44.1 g of a white solid with a yield o...
Embodiment 3
[0052] The preparation of embodiment 3 compound IVa
[0053]
[0054] Under nitrogen protection, compound IIIa (40.0g, 153.2mmol) was dissolved in 500ml of anhydrous dimethyl sulfoxide, cooled to 0°C, and TiCl was added dropwise 4 (18ml, 163.8mmol), dropwise, the solution was yellow, stirred for 5min; dropwise added N, N-diisopropylethylamine (30ml, 174mmol), dropwise, the reaction solution turned black, stirred for 1 hour; dropwise Compound IIa (32ml, 229.8mmol) was reacted at 0°C for 20 hours after dropping, and the reaction solution gradually turned yellow. Add 200ml saturated NH 4 The reaction was quenched by aqueous Cl solution, the solvent was spun off under reduced pressure, 320ml of water was added to the residue, extracted with ethyl acetate (70ml×3), the organic phases were combined, washed with water and saturated brine successively, and anhydrous MgSO 4 dry. Filtrate and concentrate under reduced pressure to obtain an oil, which was recrystallized from n-hexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com