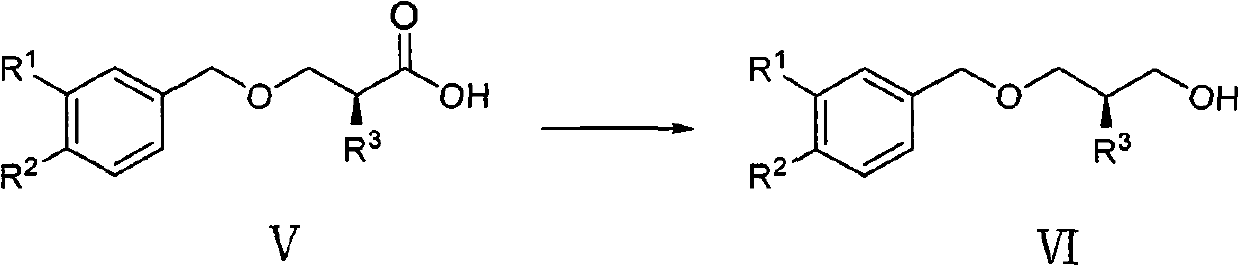
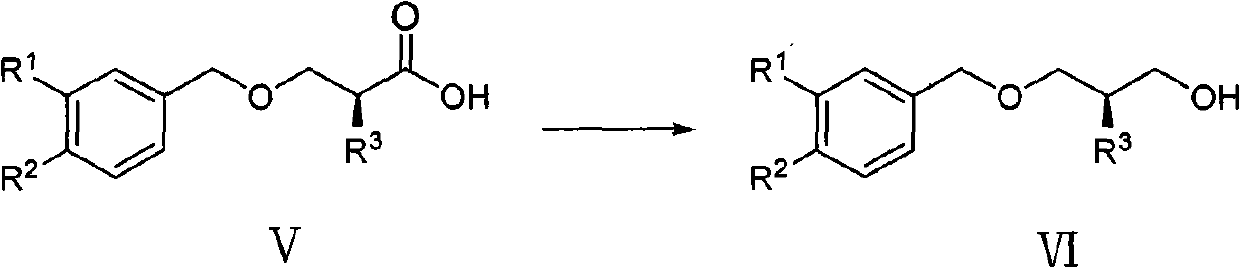
Method for preparing aliskiren intermediate
An organic solvent and straight chain technology, which is applied in the field of preparation of Alikren intermediates, can solve the problems of low reaction yield, complicated post-treatment operation and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 compound IVa
[0055]
[0056] Under nitrogen protection, compound IIIa (40.0 g, 153.2 mmol) was dissolved in 500 ml of anhydrous CH 2 Cl 2 , cooled to 0°C, dropwise added TiCl 4 (18ml, 163.8mmol), dropwise, the solution was yellow, stirred for 5min; dropwise added N, N-diisopropylethylamine (30ml, 174mmol), dropwise, the reaction solution turned black, stirred for 1 hour; dropwise Compound IIa (44ml, 316.8mmol) was reacted at 0°C for 20 hours after dropping, and the reaction solution gradually turned yellow. Add 200ml saturated NH 4 Cl aqueous solution and 320ml water, stir, separate liquid, use CH for water phase 2 Cl 2 (70ml×2) extraction, combined organic phase, washed with water and saturated brine successively, anhydrous MgSO 4 dry. Filtration and concentration under reduced pressure gave an oil (50.2 g, 85.8%), which was recrystallized from n-hexane and petroleum ether to give 46.9 g of a white solid with a yield of 80.1%...
Embodiment 2
[0061] Example 2 Preparation of Compound Va
[0062]
[0063] Under nitrogen protection, compound IVa (46.7g, 122.3mmol) was dissolved in 500ml of THF and H with a volume ratio of 3:1 2 In the mixed solution of O, when cooled to 0°C in an ice-salt bath, 30% H 2 o 2 Aqueous solution (83.6ml, 819.4mmol), after dropping, add LiOH·H 2 O (10.3g, 244.6mmol), slowly rose to room temperature, and the reaction was complete in 6 hours. Cool to below 0°C, add dropwise Na 2 SO 3 (92.5g, 733.8mmol) aqueous solution, keep the system temperature below 10°C during the dropwise addition. Filter, and wash the filter residue with cold water until the pH of the filtrate is about 12. The filtrate was evaporated to remove THF under reduced pressure at about 40°C, and the remaining aqueous phase was washed with CH 2 Cl 2 (300ml×3) After washing, discard the organic phase, adjust the pH of the aqueous phase to about 2 with 4mol / L hydrochloric acid, and then use CH 2 Cl 2 (300ml×3) extrac...
Embodiment 3
[0066] The preparation of embodiment 3 compound VIa
[0067]
[0068] Under nitrogen protection, NaBH 4 (7.0g, 183.8mmol) was suspended in 70ml of anhydrous THF, placed in an ice bath and cooled to below 10°C, and 230ml of THF solution of compound Va (27.2g, 122.5mmol) was added dropwise, stirred until no bubbles were generated, and after 5min, Add BF dropwise at the same temperature 3 ·Et 2 O (19.4ml, 153.1mmol), after dropping, stirred at room temperature, followed by TLC, and the reaction was complete in 3 hours. Cool to 0°C, slowly pour the reaction solution into 300ml of ice water, stir for 1h, extract with ethyl acetate (300ml×3), combine the organic phases, wash with saturated brine, anhydrous Na 2 SO 4 dry. After filtering and concentrating under reduced pressure, 23.6 g of oil was obtained, the yield was 92.6%, and the HPLC purity was above 97%.
[0069] Its structural identification data are as follows:
[0070] 1 H NMR (400MHz, CDCl 3 ): δ7.25-7.4(m, 5H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com