Synthetic method for mainly intermediate compounds of anti-hypertensive drug aliskiren
A synthesis method and compound technology are applied in the field of main intermediates of hypertensive drug aliskiren, and can solve the problems of high cost, high use price, cumbersome steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
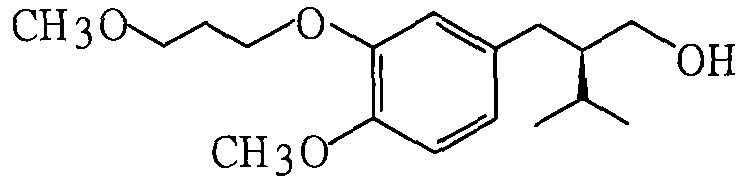
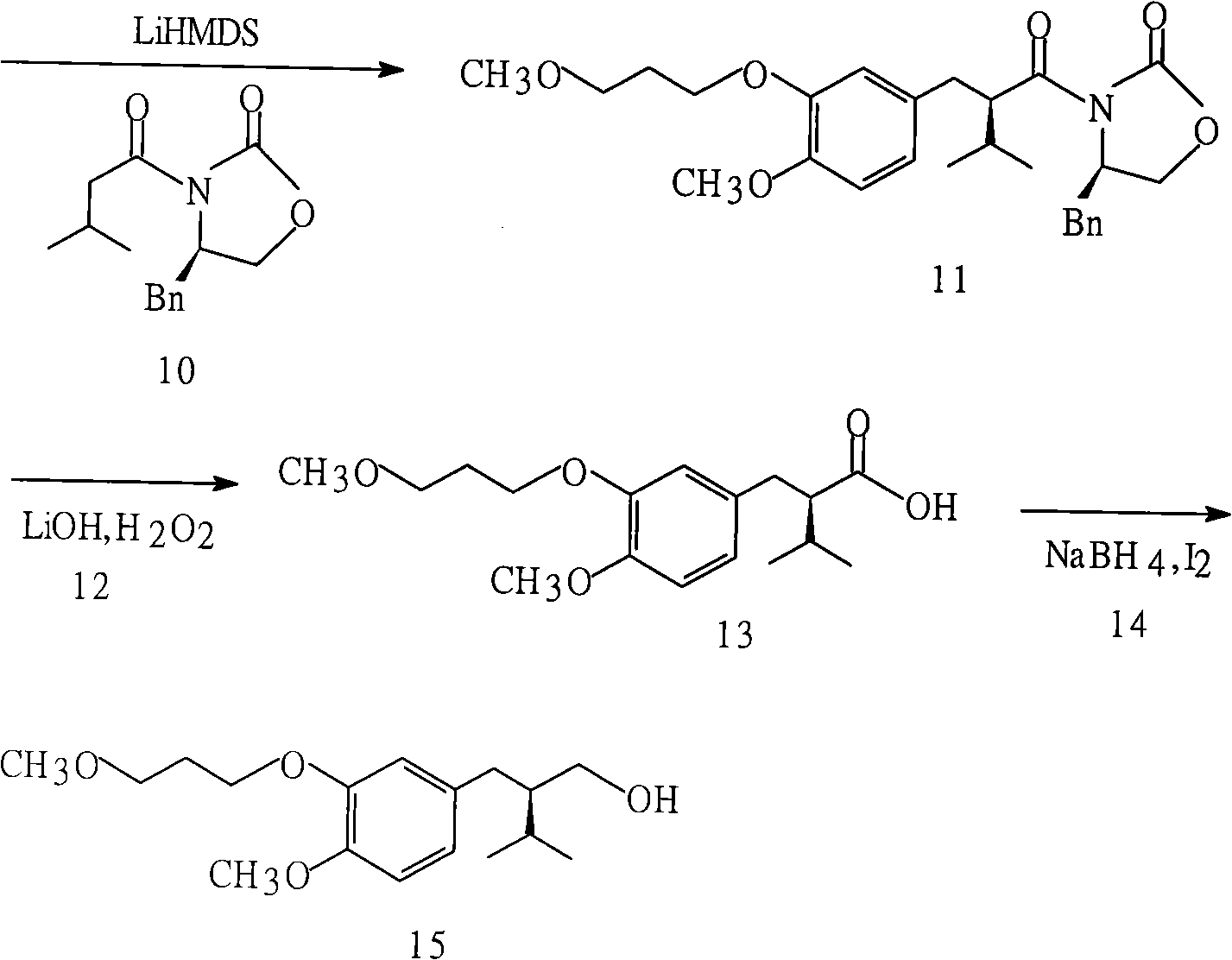
[0027] Synthesis of (2R)-3-methyl-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-1-butanol:
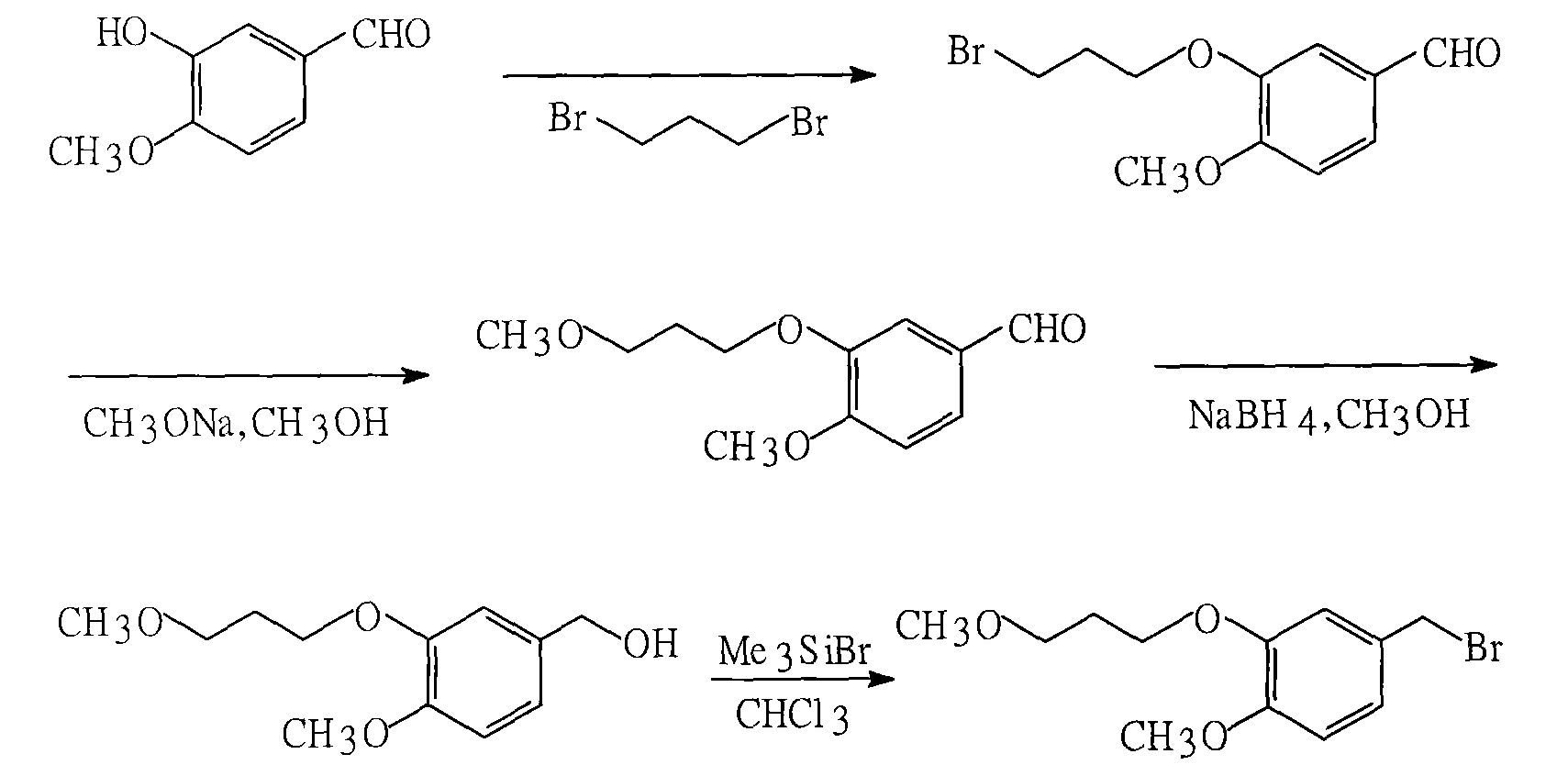
[0028] The first step: the synthesis of 4-methoxy-3-(3-methoxypropoxy)benzaldehyde
[0029] Add 600ml acetone, 152g 3-hydroxyl-4-methoxybenzaldehyde, 153g 3-methoxybromopropane, 120g anhydrous sodium carbonate in the reactor, stir, heat and reflux reaction for 6-8 hours, distill and reclaim the solvent, Add 300ml of ethyl acetate to dissolve the distillation residue, wash with 100ml of 10% aqueous sodium hydroxide solution and 100ml of water, dry with anhydrous sodium sulfate, and recover the solvent by distillation to obtain 4-methoxy-3-(3- Methoxypropoxy)benzaldehyde (yield 87%). Elemental analysis and spectroscopic data indicated that the product was the above compound:
[0030] 1 H NMR (CDCl 3 , 400MHz, TMS) δ: 9.75(s, 1H), 7.35-7.38(m, 2H), 6.89(d, 1H, J=8.0Hz), 4.09(t, 2H, J=6.6Hz), 3.86(s , 3H), 3.49(t, 2H, J=6.2Hz), 3.27(s, 3H), 2.05(m, 2H)ppm; 13 C NMR (CDCl 3 , 100MHz, TMS)...
Embodiment 2
[0043] Synthesis of (2R)-3-methyl-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-1-butanol:
[0044] The first step: the synthesis of 4-methoxy-3-(3-methoxypropoxy)benzaldehyde
[0045] Add 600ml of absolute ethanol, 152g of 3-hydroxy-4-methoxybenzaldehyde, 184g of 3-methoxybromopropane, and 120g of anhydrous potassium carbonate into the reactor, stir, heat and reflux for 6-8 hours, and recover by distillation Solvent, the distillation residue was dissolved in 300ml of ethyl acetate, washed with 100ml of 10% aqueous sodium hydroxide solution and 100ml of water, dried with anhydrous sodium sulfate, and recovered by distillation to obtain 4-methoxy-3-( 3-methoxypropoxy)benzaldehyde (90% yield). Elemental analysis and spectroscopic data indicated that the product was the above compound:
[0046] 1 H NMR (CDCl 3 , 400MHz, TMS) δ: 9.75(s, 1H), 7.35-7.38(m, 2H), 6.89(d, 1H, J=8.0Hz), 4.09(t, 2H, J=6.6Hz), 3.86(s , 3H), 3.49(t, 2H, J=6.2Hz), 3.27(s, 3H), 2.05(m, 2H)ppm; 13 C NMR (CDC...
Embodiment 3
[0059] Synthesis of (2R)-3-methyl-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-1-butanol:
[0060] The first step: the synthesis of 4-methoxy-3-(3-methoxypropoxy)benzaldehyde
[0061] Add 600ml acetonitrile, 152g 3-hydroxyl-4-methoxybenzaldehyde, 168g 3-methoxybromopropane, 120g anhydrous potassium carbonate in the reactor, stir, heat and reflux for 6-8 hours, and recycle the solvent. Add 300ml of ethyl acetate to dissolve the distillation residue, wash with 100ml of 10% aqueous sodium hydroxide solution and 100ml of water, dry with anhydrous sodium sulfate, and recover the solvent by distillation to obtain 4-methoxy-3-(3- Methoxypropoxy)benzaldehyde (95% yield). Elemental analysis and spectroscopic data indicated that the product was the above compound:
[0062] 1 H NMR (CDCl 3 , 400MHz, TMS) δ: 9.75(s, 1H), 7.35-7.38(m, 2H), 6.89(d, 1H, J=8.0Hz), 4.09(t, 2H, J=6.6Hz), 3.86(s , 3H), 3.49(t, 2H, J=6.2Hz), 3.27(s, 3H), 2.05(m, 2H)ppm; 13 C NMR (CDCl 3 , 100MHz, TMS) δ: 190.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com