Method for preparing aliskiren intermediate
An intermediate and reaction technology, which is applied in the field of preparation of non-peptide renin inhibitor aliskiren intermediates, can solve the problems of multi-hazardous chemicals, low total yield of routes, many steps, etc., and achieves reasonable cost and high yield. High efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
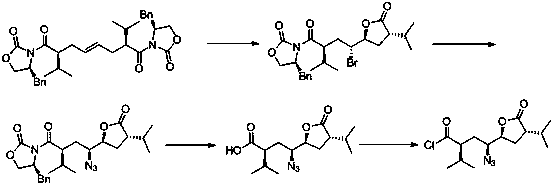
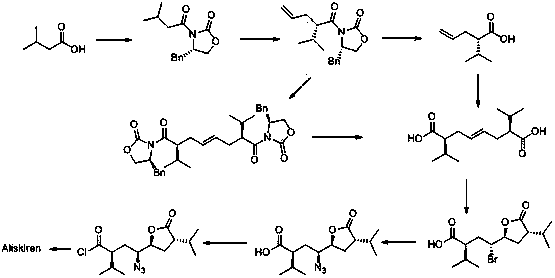
[0032] The preparation method of a kind of Aliskiren intermediate of the present invention, the synthetic route of this intermediate is:
[0033] .
Embodiment 1
[0035] Including the following synthetic steps:
[0036] (1) Synthesis of intermediate 1: under anhydrous and oxygen-free conditions, put 140mL of n-butyl (3mol / L) lithium in 600mL of tetrahydrofuran, stir, cool down to -80°C, and add dropwise; dropwise, the reaction is 30 Minutes later, 400 mL of (S)-3-(3-methylbutyryl)-4-benzyl-2-oxazolidinone (164.9 g, 0.63 mol, 3.0 eq) in tetrahydrofuran and 200 mL of trans 1,4-Dibromobutene (45.0g, 0.21mol, 1.0eq) in tetrahydrofuran; after the addition, react at -30°C for 8 hours; TLC shows that the reaction of the raw materials is complete, and quench with saturated ammonium chloride solution. Add ethyl acetate (500mL×3) for extraction; the organic layer was washed with water (500mL×2) and saturated sodium chloride solution (500mL×2), dried over anhydrous sodium sulfate, filtered with suction, and evaporated to dryness under reduced pressure to obtain 104.5 g of Yellow product. Yield: 78%.
[0037] (2) Synthesis of Intermediate 2: Put...
Embodiment 2
[0044] Including the following synthetic steps:
[0045] (1) Synthesis of intermediate 1: under anhydrous and oxygen-free conditions, put 130mL sodium hexamethyldisilazide (2moL / L) in 300mL tetrahydrofuran, stir, cool down to -80°C, and add dropwise; After dropping, add 200mL (S)-3-(3-methylbutyryl)-4-benzyl-2-oxazolidinone (98.9g, 0.34mol, 3.0eq) of tetrahydrofuran dropwise after 30 minutes of reaction solution and 100mL trans-1,4-dibromobutene (27g, 0.13mol, 1.0eq) in tetrahydrofuran; after addition, react at -30°C for 8 hours; Quenched and extracted by adding ethyl acetate (300mL×3); the organic layer was washed with water (300mL×2) and saturated sodium chloride solution (300mL×2), dried over anhydrous sodium sulfate, filtered with suction, and evaporated to dryness under reduced pressure. 60.3 g of pale yellow product was obtained. Yield: 75%.
[0046] (2) Synthesis of intermediate 2: Put 54g of intermediate 1 (0.09mol, 1.0eq) in 350mL of tetrahydrofuran, stir, then add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com