Preparation method of 4-chloromethyl-5-methyl-1, 3-dioxole-2-ketone
A technology of dioxole and chloromethyl, which is applied in the field of drug synthesis, can solve the problems of increasing the difficulty of environmental protection and high irritation, and achieve the effects of low difficulty in wastewater treatment, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
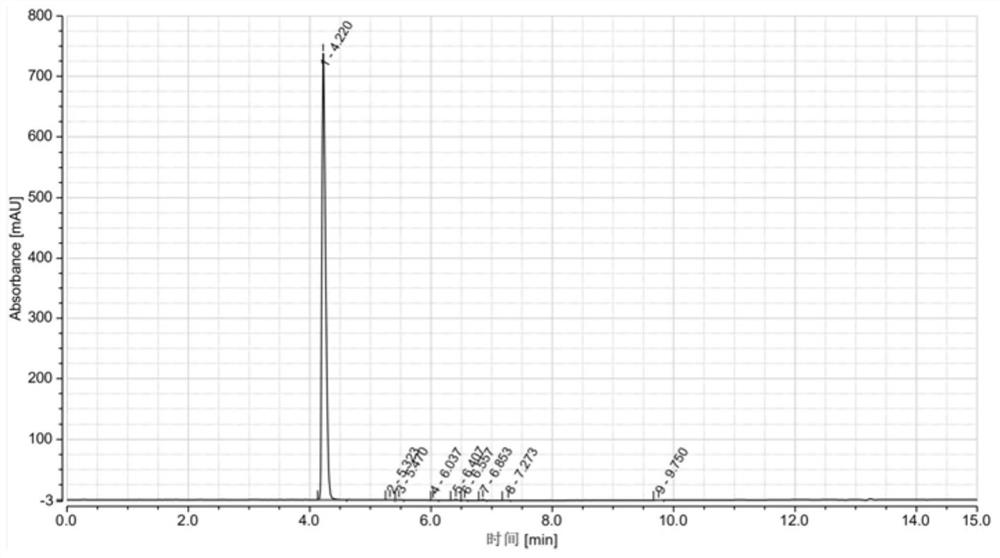
[0034] Put 50gDMDO and 300mL toluene into a dry three-necked flask, and stir evenly; at 0-10°C, add 87.3g of phosphorus oxychloride dropwise into the three-necked flask, and control the dropping time for 1.5h. Insulate at ℃ for 1h; heat up to 90°C and reflux, keep the temperature for 5h, cool to room temperature, and obtain the reaction product; the reaction product is stirred and washed with 3wt.% potassium carbonate aqueous solution, and the organic phase is separated with a separatory funnel after washing, and washed several times in this way Until the pH of the mixed solution is neutral, the washed organic phase is obtained; the washed organic phase is distilled off under reduced pressure to remove the solvent, and distilled at 91-93° C. under a vacuum of 2 mmHg to obtain 65.1 g of DMDO-Cl as a colorless liquid. Product purity 99.412%, yield 85.7%, see HPLC figure figure 1 , see Table 1 for HPLC data.
[0035] The HPLC data table of table 1 embodiment 1
[0036] ...
Embodiment 2
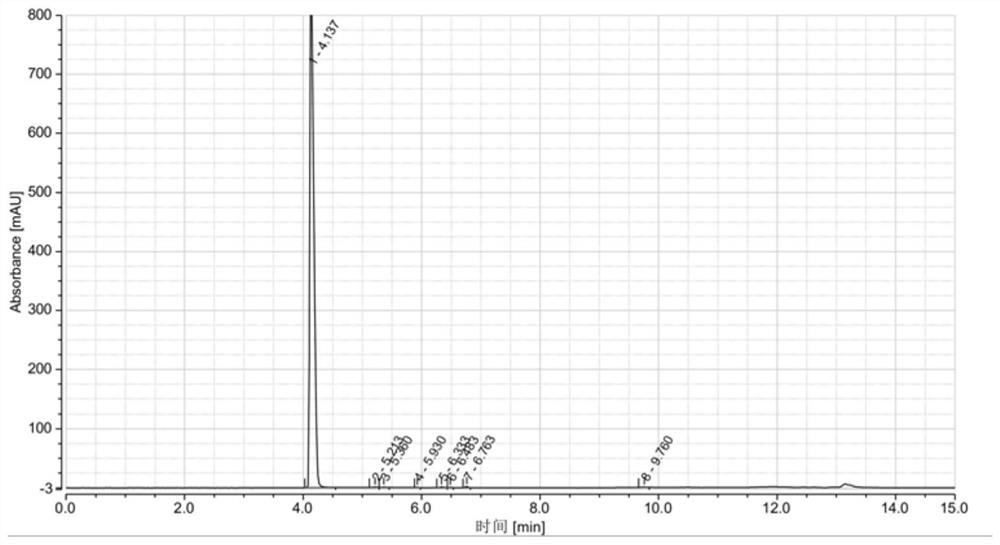
[0038] Put 50gDMDO and 400mL toluene into a dry three-necked flask, and stir evenly; at 10-20°C, add 80.5g of phosphorus oxychloride dropwise into the three-necked flask, and control the dropping time for 1h. After the dropwise addition, 10-20°C Keep warm for 0.5h; heat up to 100°C to reflux, keep warm for 6h, cool to room temperature, and get the reaction product. The reaction product was stirred and washed with 5wt.% potassium carbonate aqueous solution, and after washing, the organic phase was separated with a separatory funnel, and washed several times in this way until the pH of the mixed solution was neutral, and the washed organic phase was obtained. The washed organic phase was distilled off under reduced pressure to remove the solvent, and distilled at 91-93° C. under a vacuum of 2 mmHg to obtain a colorless liquid of DMDO-Cl. Product purity 99.368%, yield 82.5%, see HPLC figure figure 2 , see Table 2 for HPLC data.
[0039] The HPLC data table of table 2 embodimen...
Embodiment 3
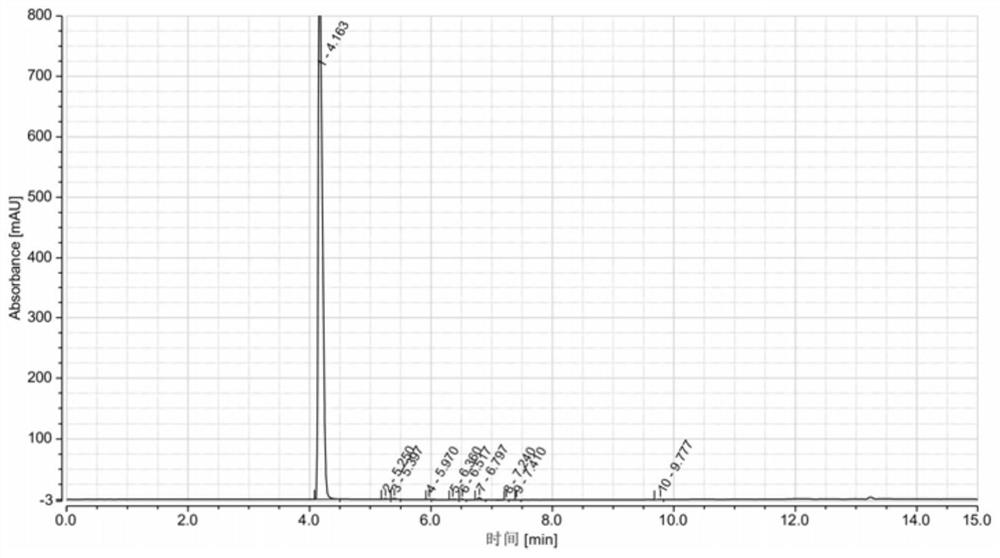
[0042] Put 50gDMDO and 300mL toluene into a dry three-necked flask, and stir evenly; at 25-35°C, add 73.9g of phosphorus oxychloride dropwise into the three-necked flask, and control the dropping time for 1h, after the dropwise addition is completed, 25-35°C Keep warm for 0.5h; heat up to 110°C to reflux, keep warm for 4h, cool to room temperature, and get the reaction product. The reaction product was stirred and washed with 1wt.% potassium carbonate aqueous solution, and after washing, the organic phase was separated with a separatory funnel, and washed several times in this way until the pH of the mixed solution was neutral, and the washed organic phase was obtained. The washed organic phase was distilled off under reduced pressure to remove the solvent, and distilled at 91-93° C. under a vacuum of 2 mmHg to obtain a colorless liquid of DMDO-Cl. Product purity 99.129%, yield 81.2%, see HPLC figure image 3 , see Table 3 for HPLC data.
[0043] The HPLC data table of table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com