Pharmaceutical composition for treating hypertension and cardiovascular disease
A composition and cardiovascular technology, applied in the field of medicine, can solve the problems of not taking into account the regularity of blood pressure changes in patients, low blood pressure, and unsatisfactory blood pressure control, and achieve the effects of eliminating adverse reactions, reducing blood volume, and avoiding photolysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1-13
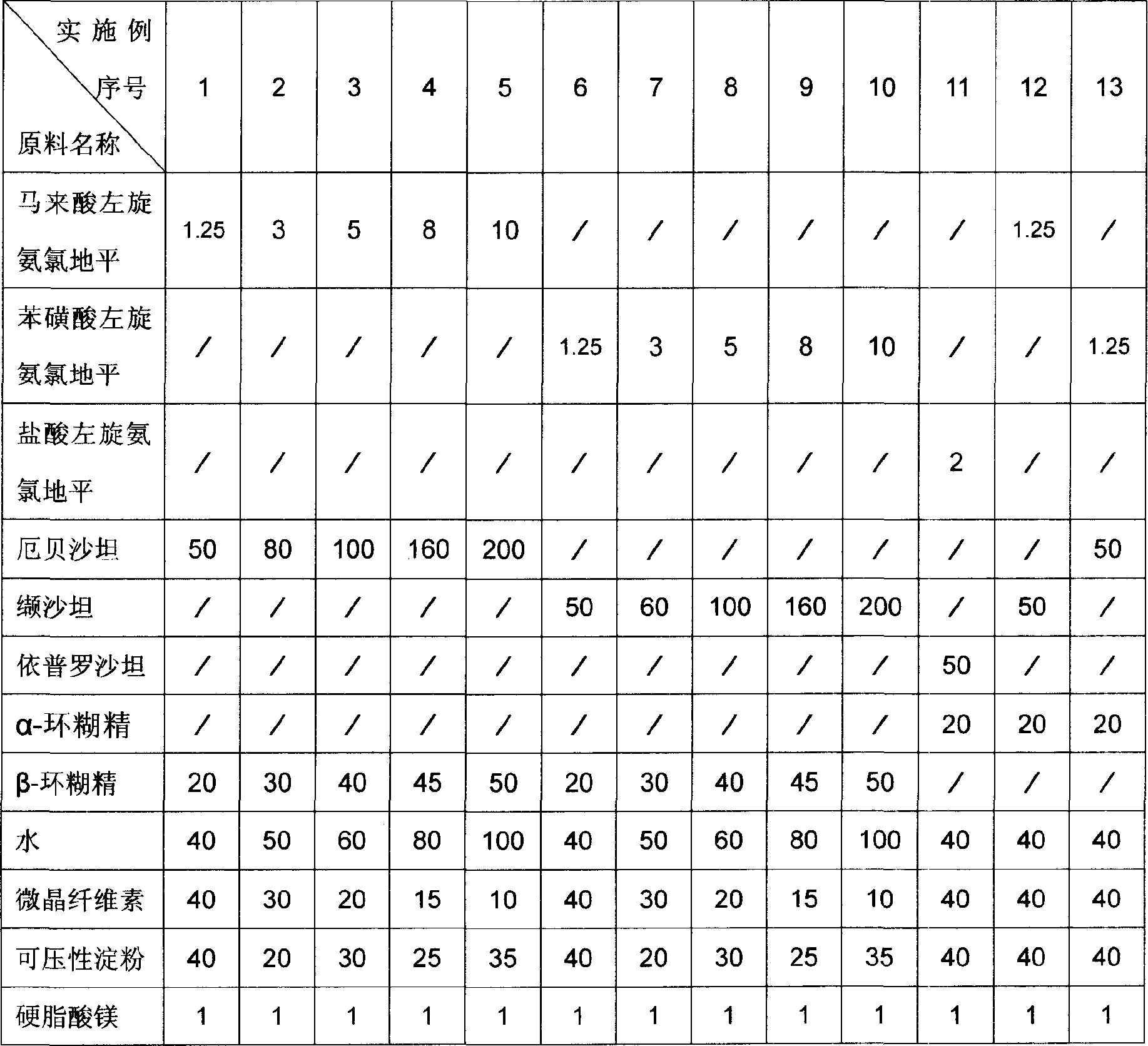
[0022] The dosage forms of the pharmaceutical compositions of Examples 1 to 13 are tablets, and the raw material formulations of each example are the amount for preparing 1000 tablets, and the unit is gram. The raw material formula of embodiment 1 to embodiment 13 is provided by table 1.
[0023] The preparation method of Example 1 to Example 13: Grinding levamlodipine salt, angiotensin II receptor antagonist, corresponding cyclodextrin and water efficiently and quickly for 1 hour, then drying in vacuum at 40°C, pulverizing, passing through 1000 Mesh sieve, then add the remaining materials, mix in a mixer for 10 minutes, press into tablets, and pack after passing the test.
[0024] Usage: take orally, once a day, one tablet each time, take it in the morning.
Embodiment 14-16
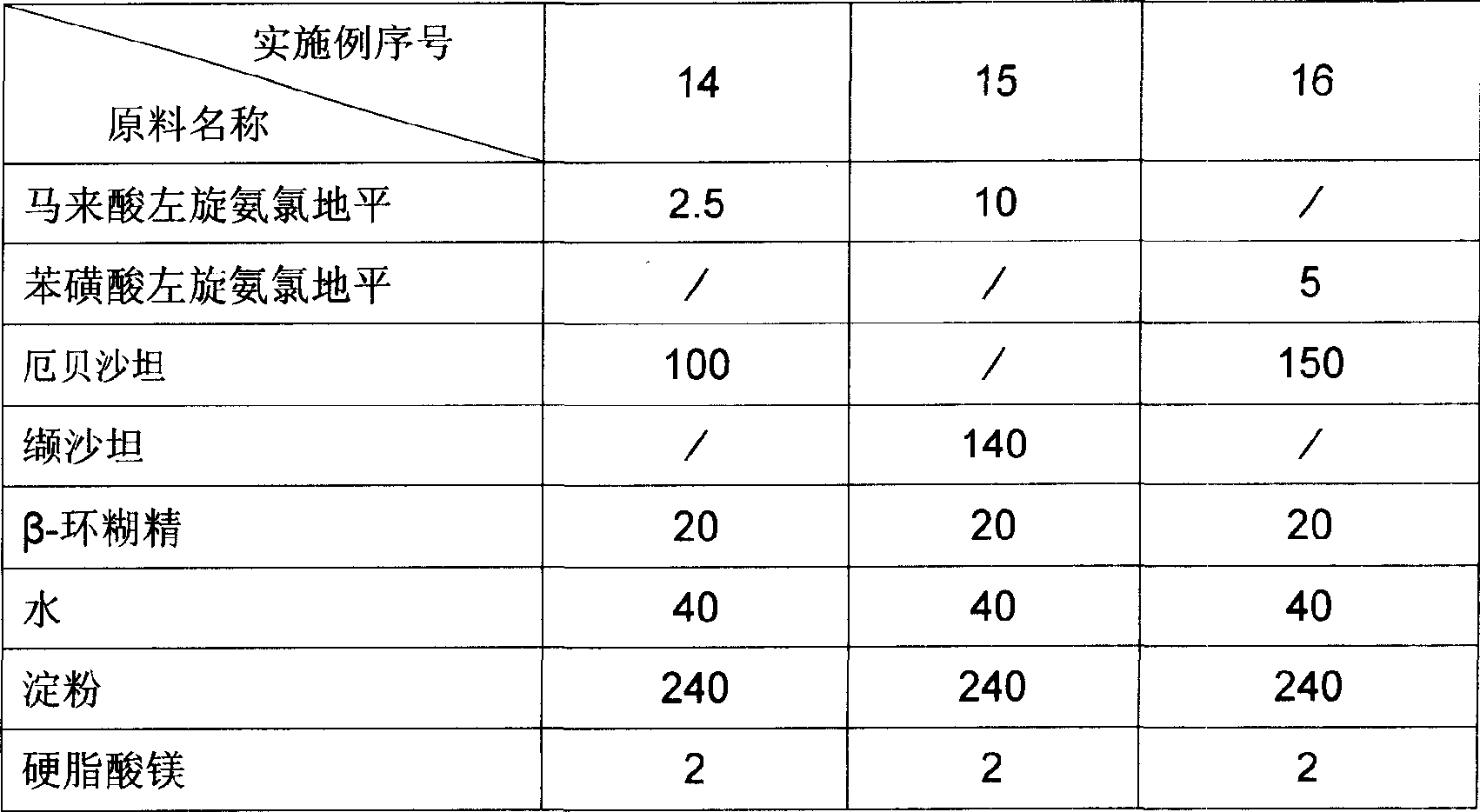
[0026] The dosage forms of the pharmaceutical compositions of Examples 14 to 16 are capsules, and the raw material formulations of each example are the amount of medicine in 1000 capsules, and the unit is gram. The raw material formula of embodiment 14 to embodiment 16 is provided by table 2.
[0027] The preparation method of Example 14 to Example 16: Preparation method: Grind levamlodipine salt, angiotensin II receptor antagonist, cyclodextrin and water efficiently and rapidly for 1 hour, then dry in vacuum at 40° C., pulverize, pass 1000-mesh sieve, then add the remaining materials, mix in a mixer for 10 minutes, pack into capsules, and pack after passing the test.
[0028] Usage: Orally, once a day, one tablet each time, in the morning.
Embodiment 17-19
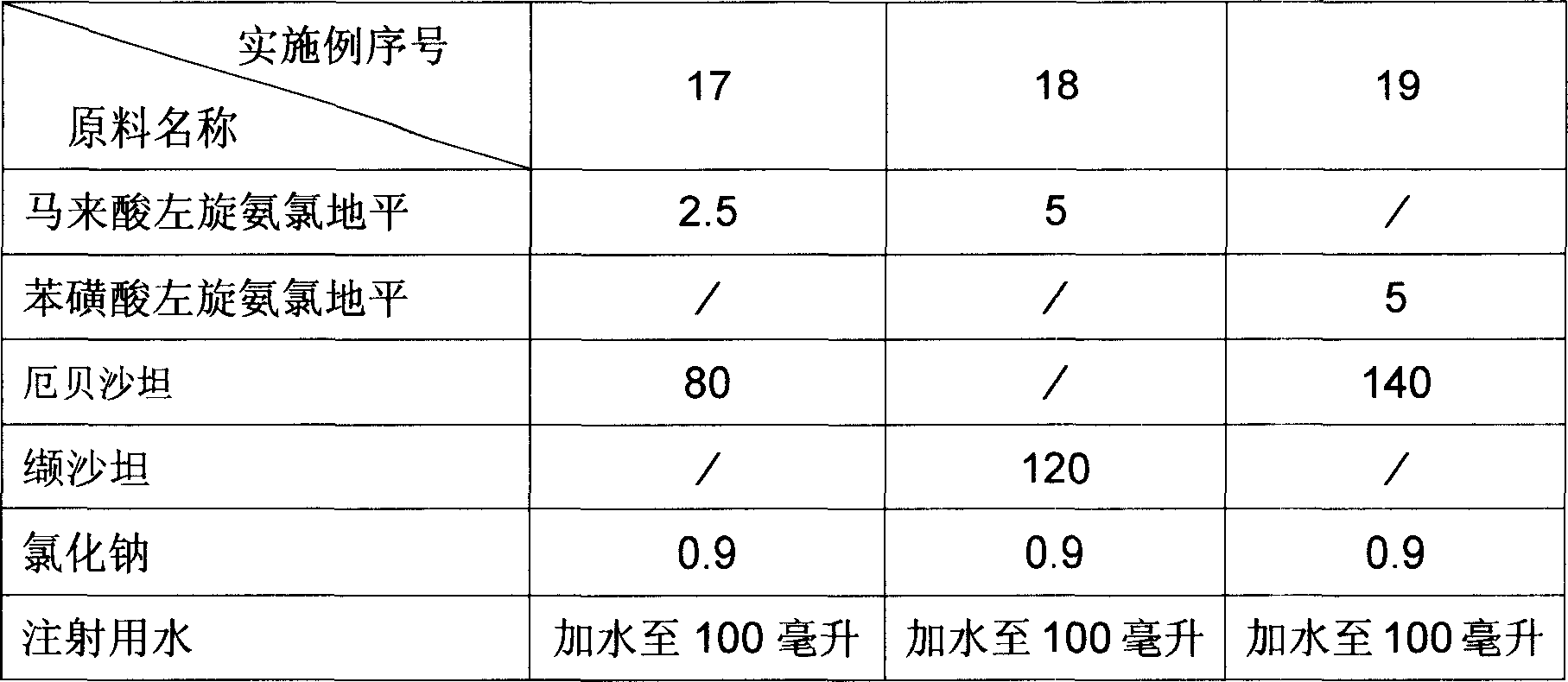
[0030]The dosage forms of the pharmaceutical compositions of Examples 17 to 19 are injections, and the raw material formulations of each example are the amount for preparing 100 injections, and the unit is gram. The raw material formula of embodiment 17 to embodiment 19 is provided by table 3.
[0031] The preparation method of Example 17 to Example 19: Dissolve sodium chloride in water for injection, add levamlodipine salt, angiotensin II receptor antagonist, further add water for injection after dissolving, adjust the volume to the required concentration , and then the solution is subjected to sterile filtration, and filled into ampoules or oral liquid bottles under aseptic conditions.
[0032] How to use: Inject, once a day, one each time, use in the morning.
[0033] Table 1 Unit: Gram
[0034]
[0035] Table 2 Unit: Gram
[0036]
[0037] Table 3 Unit: Gram
[0038]
[0039] Medicine of the present invention has been tested, and 200 routine hypertensive patie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com