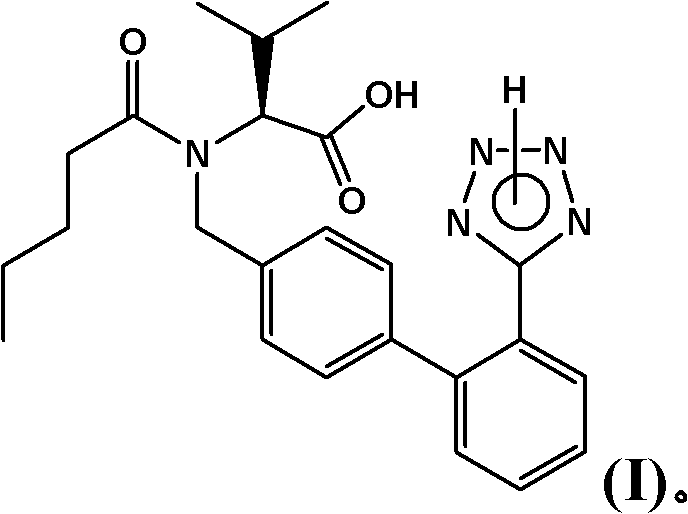
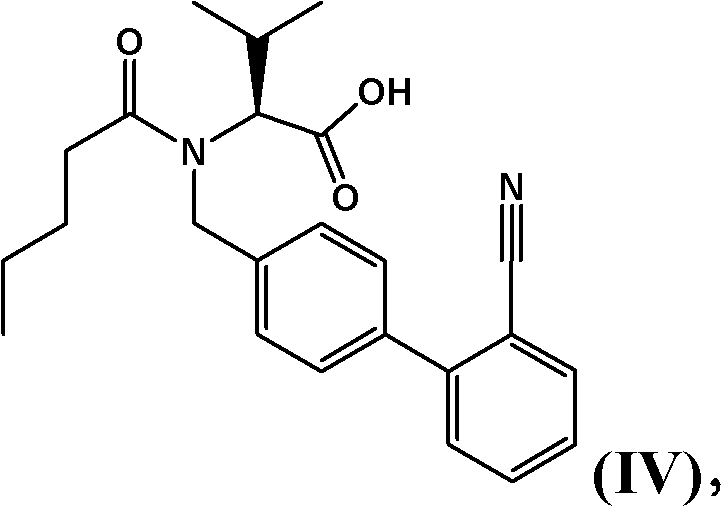
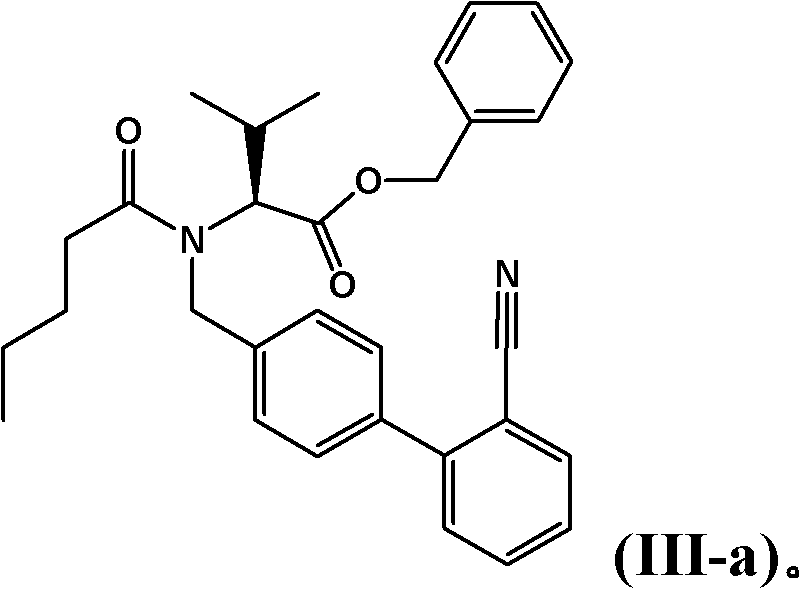
Process for the manufacture of organic compounds
A compound and halide technology, applied in the field of synthesizing valsartan, can solve the problems such as the reduction of the yield of the desired product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment II
[0336] Embodiment II:Preparation of "acid-nitriles" via cleavage of benzyl esters of "benzyl-nitriles" with diethylaluminum chloride
[0337]
[0338] To a solution of 2.41 g (5 mmol) of "benzyl ester-nitrile" in 10 ml of anhydrous toluene was added a 1.8 M solution of diethylaluminum chloride in toluene (12.5 ml, 22.5 mmol) via syringe at room temperature under nitrogen and stirring. ). The addition was exothermic. After fully joining Et 2 After the AlCl solution the reaction mixture was warmed to 50 °C. After 2 hours the reaction mixture was cooled to 0°C and then quenched by the slow addition of 20 ml of 2M hydrochloric acid. Termination was largely exothermic and gas evolution was observed. The phases were separated and the organic phase was washed with 3 x 20 ml of water. The organic phase was evaporated in vacuo to give an oil. Spectral data is identical with embodiment 1.
[0339] Reference Example III: Preparation of valsartan via a one-pot process using ...
Embodiment IV
[0345] Embodiment IV: Preparation of valsartan: via the use of diethylaluminum chloride (for ester cleavage) and diethylaluminum azide (for cycloaddition) produced in situ by reacting diethylaluminum chloride with sodium azide into reaction) "one-pot method"
[0346]
[0347] 60 g of (S)-2-[(2'-cyanobiphenyl-4-ylmethyl)pentanoylamino]-3-methylbutanoic acid benzyl ester ("benzyl ester-nitrile" ) in xylene (67.1 mmol) was mixed with 33 g of diethylaluminum chloride (265.5 mmol) and stirred for 1 hour, the mixture was allowed to warm up to 50° C. by intermittent cooling. 8.82 g of sodium azide (134.3 mmol) were added and the mixture was heated to 110°C. After 4 hours the reaction mixture was carefully transferred to a flask containing 201.6 g of 12% by weight aqueous NaOH while raising the temperature of the mixture to 50°C. The upper organic phase was discarded, and the aqueous phase was washed with 60 g of xylene.
[0348] The oily phase containing product was separated...
Embodiment V
[0350] Embodiment V: Esterification of intermediate "acid-nitrile" with 3-methyl-1-(p-tolyl)triazene
[0351]
[0352] 9.8 g (65.56 mmol) of 3-methyl-1-(p-tolyl)triazene were dissolved in 100 ml of dichloromethane. To this solution was added 24.4 g (59.6 mmol) of a solution of "acid-nitrile" dissolved in 200 ml of dichloromethane via the dropping funnel within 45 minutes at room temperature with stirring. After 2 hours the reaction mixture was treated with 100 mL of 1M hydrochloric acid. The organic phase was washed with water, dried over sodium sulfate and evaporated in vacuo to give a light yellow oil which was pure according to HPLC and H-NMR analysis.
[0353] 1 H-NMR (400MHz, CDCl 3 );δ H (ppm), a mixture of rotamers (6:4) at room temperature. 0.82-0.95 (5H, m, -CH 3 , 60%), 0.95-1.03 (4H, m, -CH 3 , 40%), 1.25-1.37 (1H, m, -CH-, 40%), 1.40-1.49 (1H, m, -CH-, 60%), 1.60-1.70 (1H, m, -CH-, 60 %), 1.70-1.82 (1H, m, -CH-, 40%), 2.22-2.65 (3H, br, copl.m, -CH- an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com