Compound antihypertensive preparation and application thereof
A technology of high blood pressure and receptor antagonists, applied in pill delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., to achieve the effect of reducing adverse reactions, improving compliance, and widening the population of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
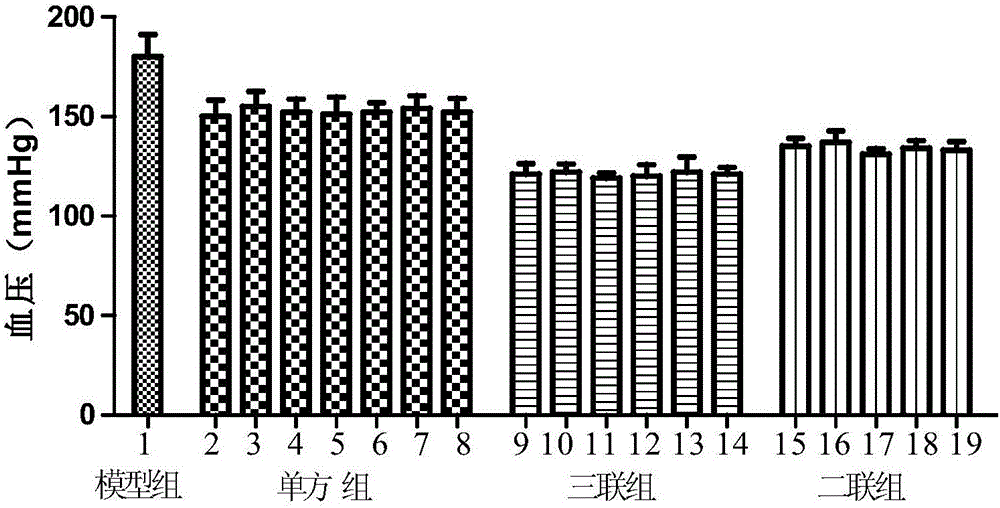
[0047] Example 1: Antihypertensive Effect Experiment of Triple Drug Combination on Hypertension in Spontaneously Hypertensive Rats
[0048] 1. Experimental animals and experimental groups
[0049] 190 spontaneously hypertensive male rats, weighing 250g±20g, were randomly divided into 19 groups, 10 rats in each group, after being fed for one week.
[0050] (1) Model control group: intragastric administration of the same volume of normal saline;
[0051] (2) Metolazone group: 0.05mg / kg / d
[0052] (3) Hydrochlorothiazide: 1.2mg / kg / d
[0053] (4) Valsartan group: 7.5mg / kg / d
[0054] (5) Olmesartan group: 4mg / kg / d
[0055] (6) Amlodipine group: 1mg / kg / d
[0056] (7) Lacidipine group: 0.4mg / kg / d
[0057] (8) Felodipine group: 0.5mg / kg / d
[0058] (9) Metolazone + valsartan + amlodipine: 0.05mg / kg / d+7.5mg / kg / d+1mg / kg / d
[0059] (10) Metolazone + valsartan + lacidipine: 0.05mg / kg / d+7.5mg / kg / d+0.4mg / kg / d
[0060] (11) Metolazone + valsartan + felodipine: 0.05mg / kg / d+7.5mg / kg / d+...
Embodiment 2
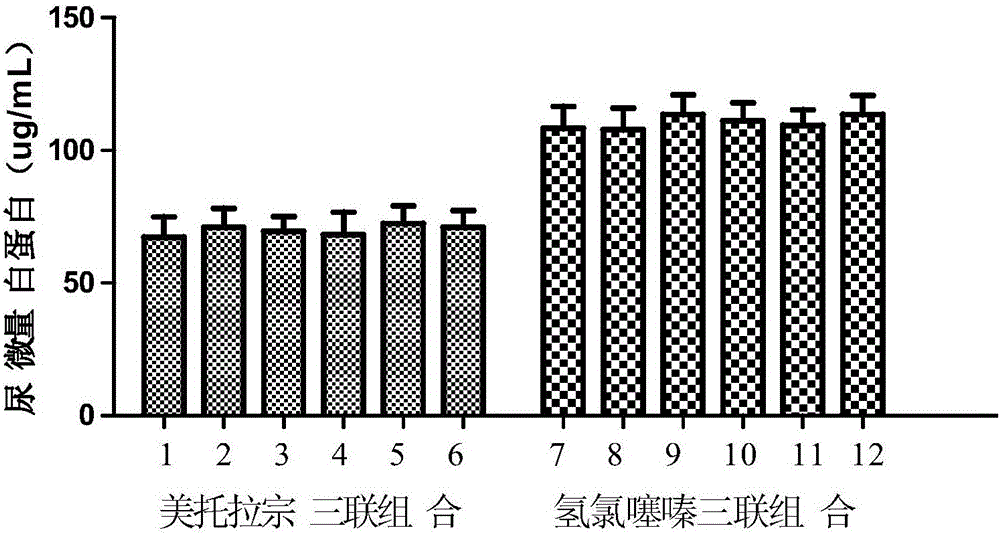
[0075] Example 2: Compared with the triple drug combination containing hydrochlorothiazide, the triple drug combination of the present invention has an antihypertensive effect on spontaneously hypertensive rats and an experiment on kidney damage
[0076] 1. Experimental animals and experimental groups
[0077] 120 spontaneously hypertensive male rats, weighing 250g±20g, were randomly divided into 12 groups, 10 rats in each group, after being fed for one week.
[0078] (1) Metolazone + valsartan + amlodipine: 0.05mg / kg / d+7.5mg / kg / d+1mg / kg / d
[0079] (2) Metolazone + valsartan + lacidipine: 0.05mg / kg / d+7.5mg / kg / d+0.4mg / kg / d
[0080] (3) Metolazone + valsartan + felodipine: 0.05mg / kg / d+7.5mg / kg / d+0.5mg / kg / d
[0081] (4) Metolazone + Olmesartan + Amlodipine: 0.05mg / kg / d+4mg / kg / d+1mg / kg / d
[0082] (5) Metolazone + Olmesartan + Lacidipine: 0.05mg / kg / d+4mg / kg / d+0.4mg / kg / d
[0083] (6) Metolazone + Olmesartan + Felodipine: 0.05mg / kg / d+4mg / kg / d+0.5mg / kg / d
[0084] (7) Hydrochlorot...
Embodiment 3
[0126] Embodiment 3, the preparation of compound pharmaceutical composition 3 (tablet) (in 1000 pieces)
[0127] Tablet prescription:
[0128]
[0129] Coating prescription:
[0130] Opadry Coating Powder 9g
[0131] Purified water 60g
[0132] Preparation:
[0133] (1) Weigh the prescribed amount of valsartan, metolazone, and amlodipine, pulverize them through an 80-mesh sieve, and mix them uniformly by using the method of equal increments.
[0134] (2) Weigh the microcrystalline cellulose, starch, croscarmellose sodium, and micropowder silica gel of the prescribed amount, pulverize them through a 60-mesh sieve, and mix them evenly.
[0135] (3) Mix (1) and (2) evenly, add 95% ethanol to make a soft material, granulate with an 18-mesh sieve, and dry at 50°C.
[0136] (4) After the granules are dried, the granules are sized through a 20-mesh sieve, then magnesium stearate is added, and the mixture is evenly mixed to obtain an intermediate.
[0137] (5) Check the inter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com