Combined preparation for the treatment of cardiovascular diseases based on chronotherapy theory
A preparation, angiotensin technology, applied in the field of designed combined preparations, can solve the problems of various pathophysiological causes of uncomfortable hypertension, difficulty in preventing the deterioration of complications, and no consideration of drug characteristics, so as to improve medication compliance, reduce Excellent blood pressure effect and the effect of saving prescription time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
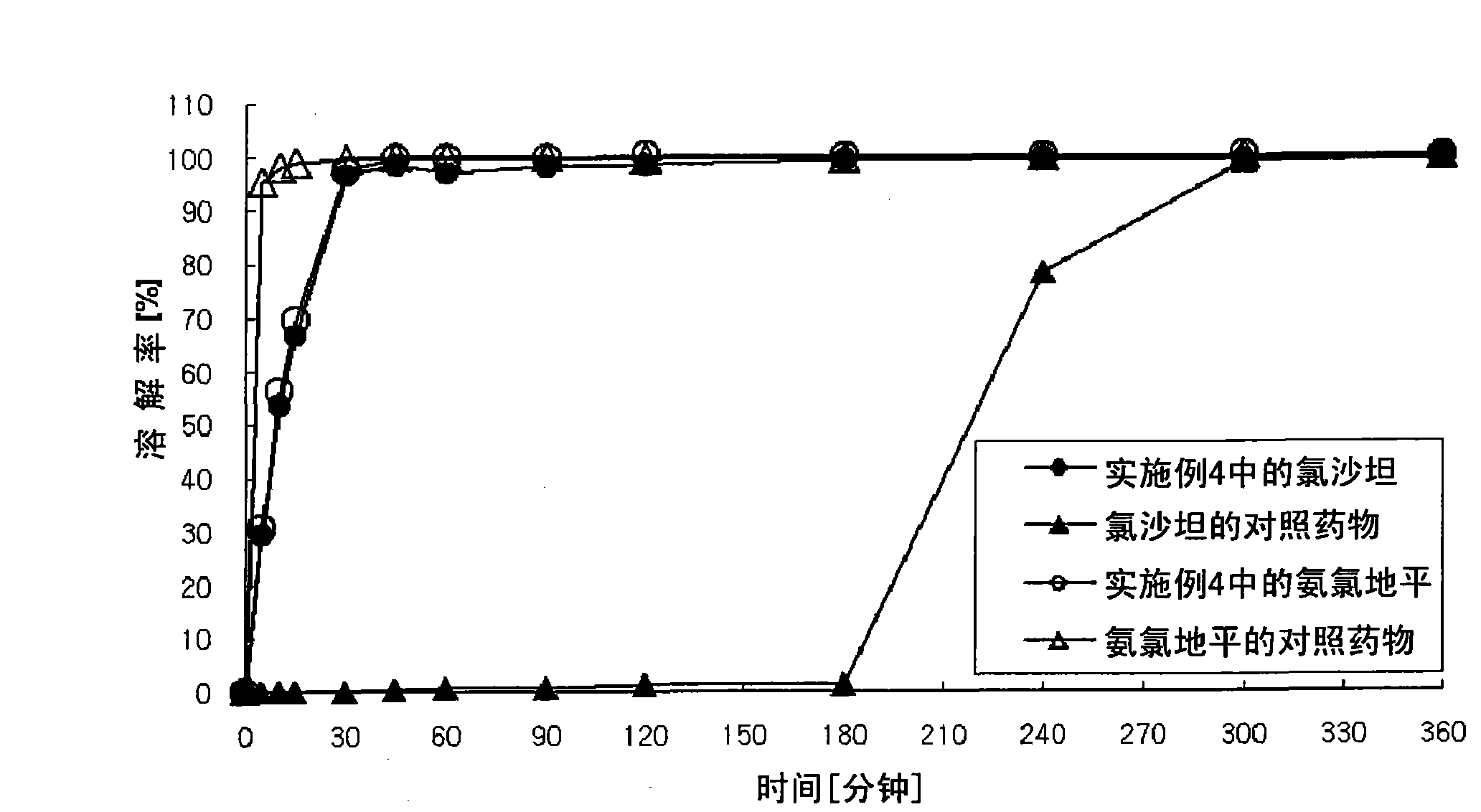
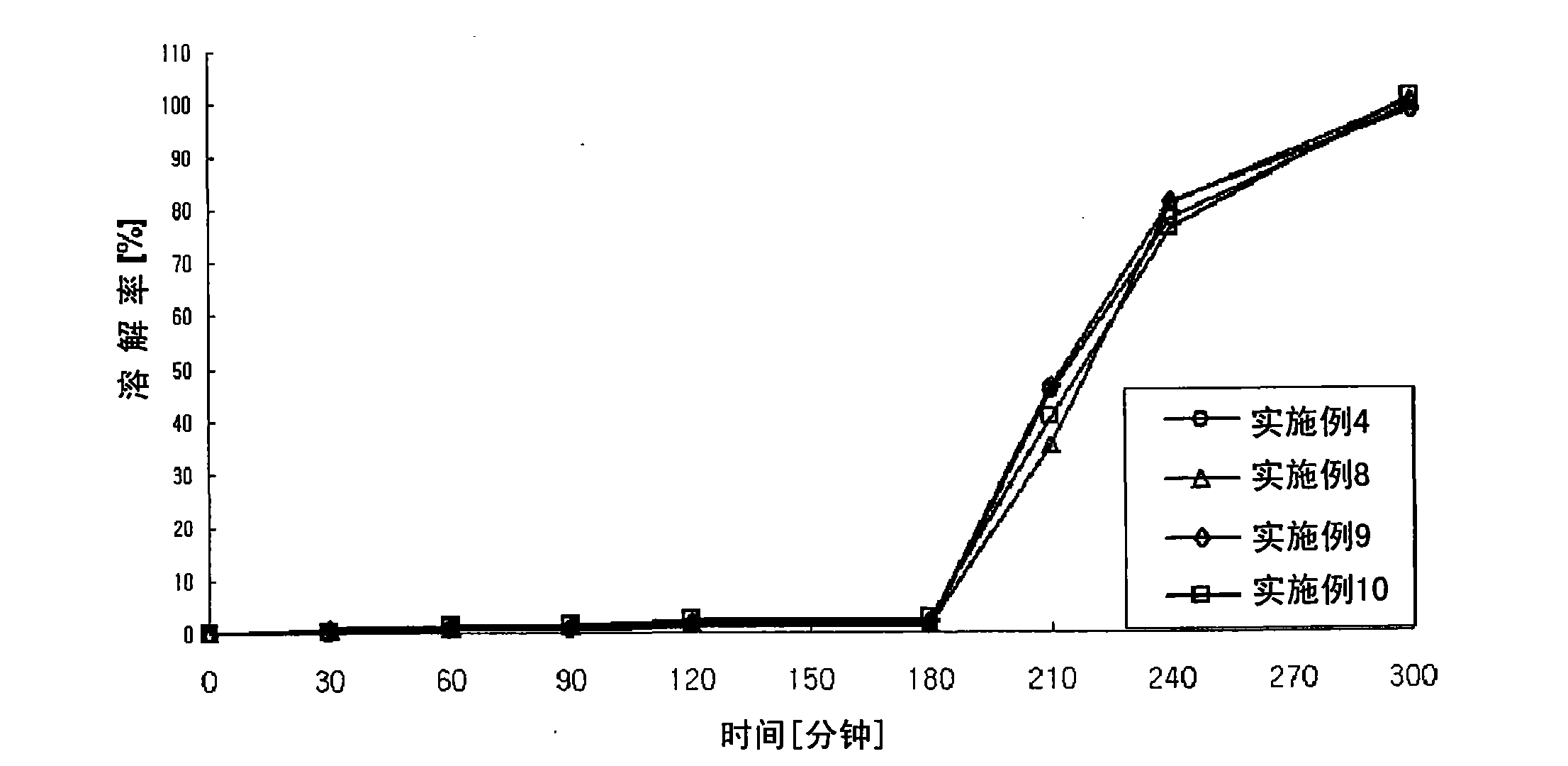
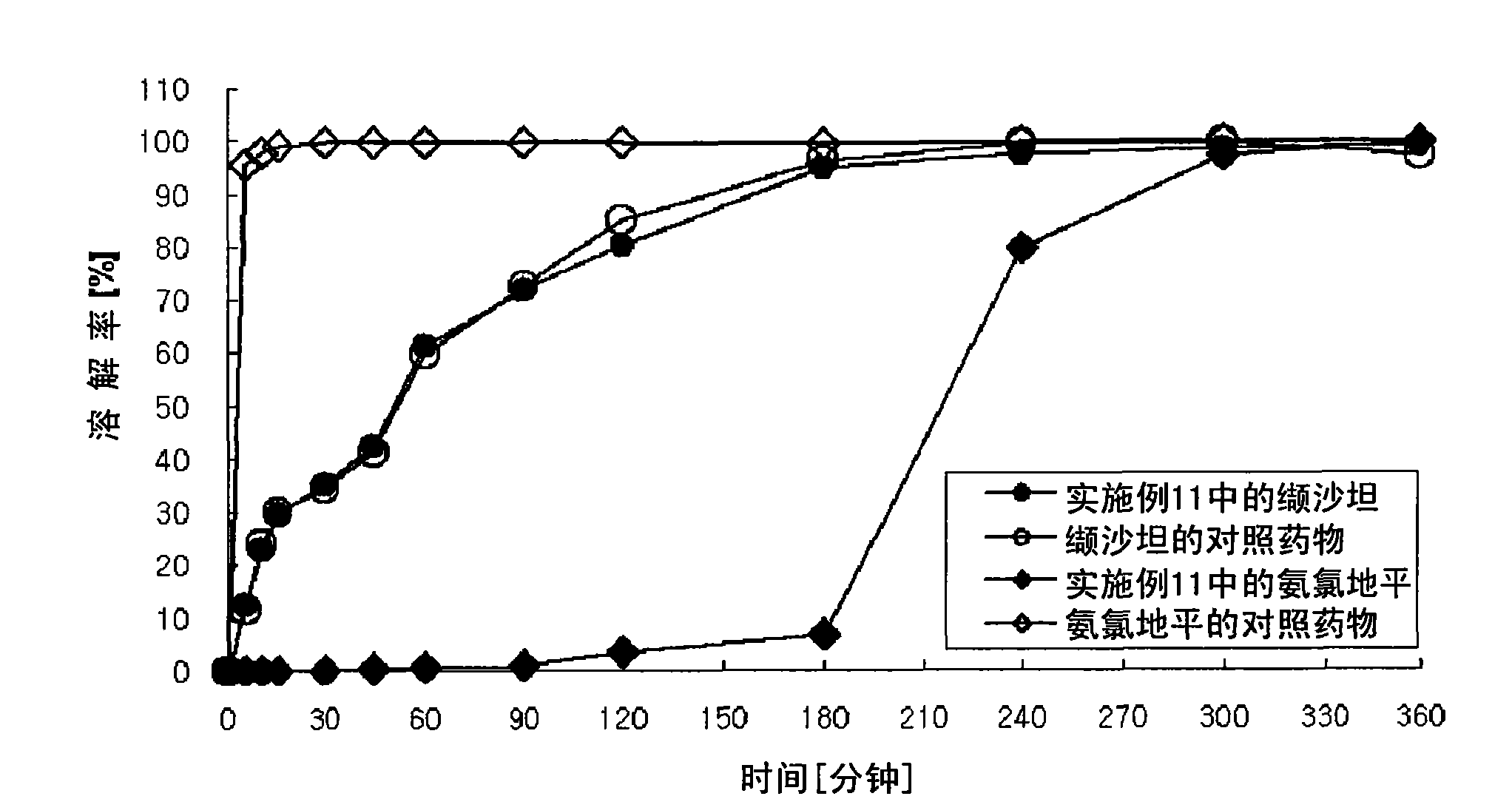
Image
Examples
preparation example Construction
[0108] A. Preparation of Tablets
[0109] Tablets of uniform weight are prepared by mixing the granules prepared in the first step, optionally coated with a release-controlling material, with the granules prepared in the second step, followed by compression. The resulting tablets may require film coatings to improve stability or shape.
[0110] B. Preparation of Multilayer Tablets
[0111] The granules prepared in the first step are optionally coated with a controlled release material and dried. The dried granules are compressed with the granules prepared in the second step by using a multi-layer tablet press machine, thereby obtaining a bi-layer tablet. Optionally, a three-layer or more-layer tablet can also be prepared by further adding a release aid layer on the bi-layer tablet. A coated multilayer tablet can be prepared by coating said multilayer tablet.
[0112] C. Preparation of Core Tablets
[0113] The coated tablets or granules prepared in the first step are opti...
Embodiment 1
[0132] Embodiment 1: the preparation of single pill
[0133] (1) Preparation of Losartan Potassium Granules
[0134] Predetermined amounts of losartan potassium, lactose and microcrystalline cellulose as shown in Table 4 were sieved through a No. 35 sieve and mixed for 5 minutes with a double cone mixer. The mixture was placed in a fluidized bed granulator (GPCG 1: Glatt), sprayed with a binder solution (aqueous solution of hydroxypropylmethylcellulose) to prepare granules, and dried. The granules are added with Carbomer 71G powder, mixed with magnesium stearate using a double cone mixer. The resulting mixture was compressed with a rotary tablet press (MRC-33: Sejong) at a speed of 30 revolutions per minute to provide tablets having a hardness of 7-9 kp, a thickness of 3.0 mm, and a diameter of 5.5 mm.
[0135] Predetermined amounts of losartan potassium, lactose and microcrystalline cellulose as shown in Table 4 were sieved through a No. 35 sieve and mixed for 5 minutes wit...
Embodiment 2
[0143] As shown in Table 4, tablets were prepared as described in Example 1, except that instead of using cellulose acetate (32% of acetal groups), only cellulose acetate (32% of acetal groups), cellulose acetate (32% of acetal groups), group 39.8%) and hydroxypropyl methylcellulose.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com