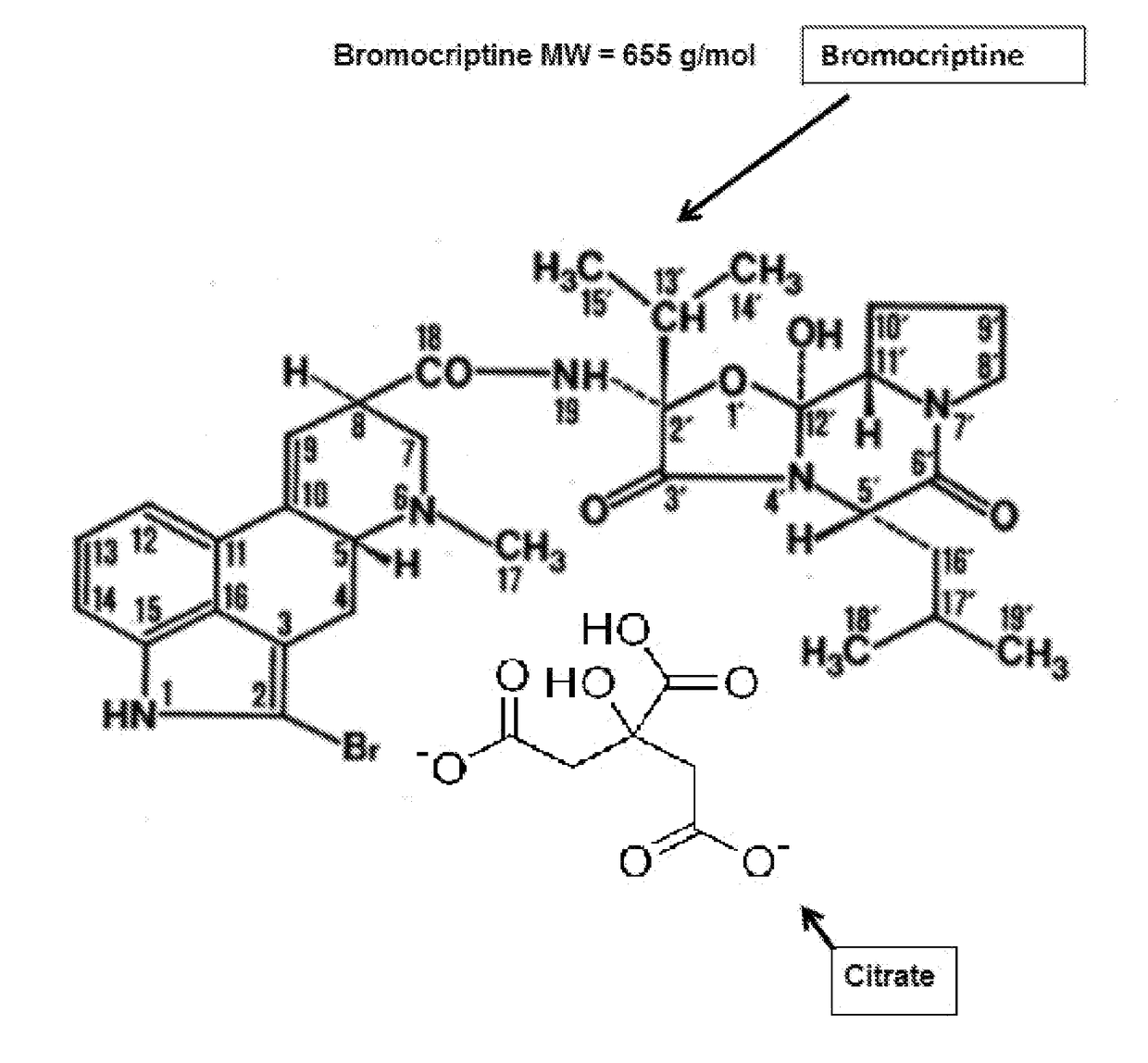
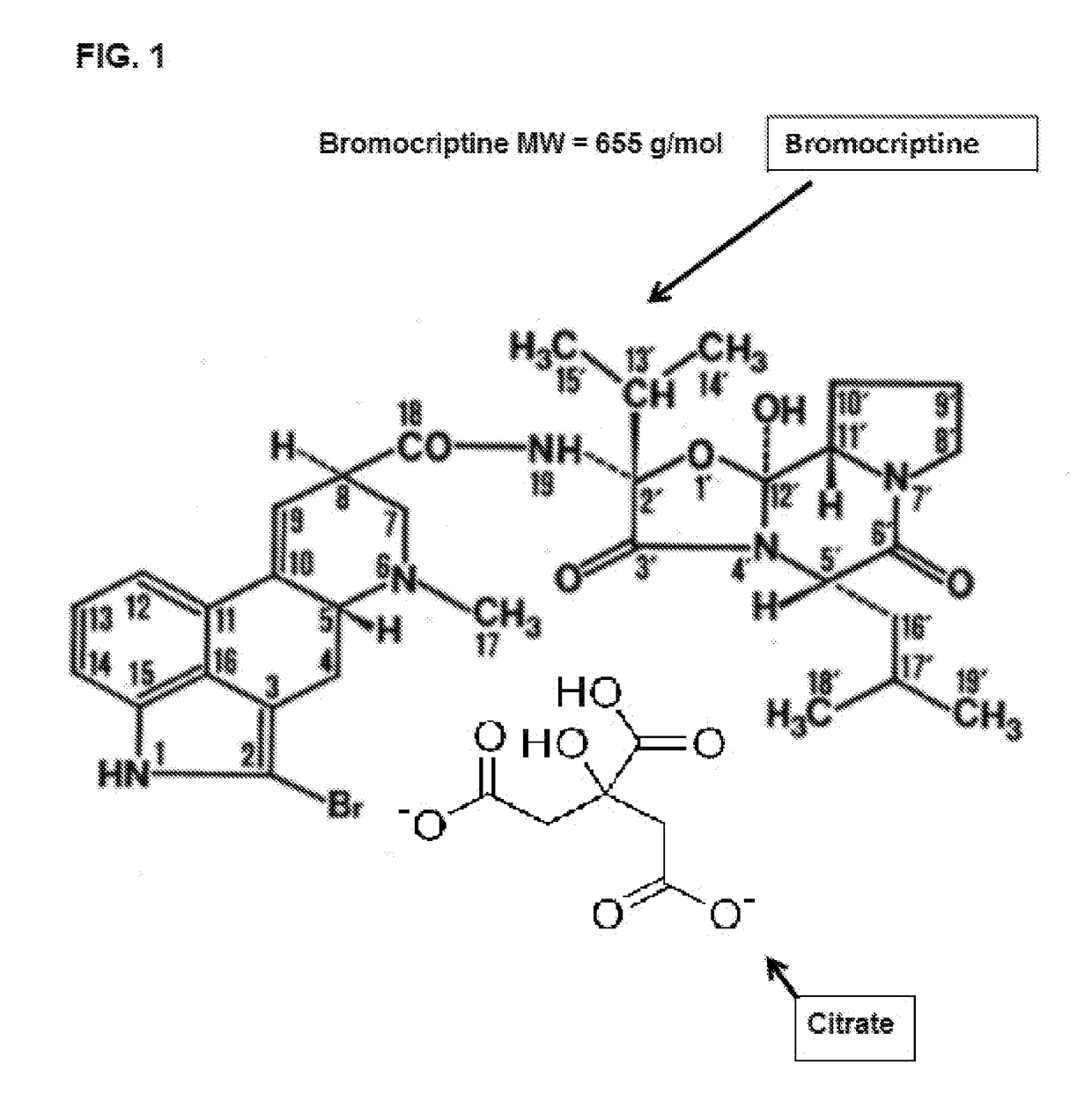
Composition and Method for Treating Metabolic Disorders
a metabolic disorder and composition technology, applied in the field of bromocriptine citrate synthesis, can solve the problems of insufficient or less than fully effective insulin amount, adverse effects of diabetes on the way the body uses sugar, and elevated glucose in the blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0051]Citric acid was dissolved, in separate reaction vessels, in one of either methanol, ethanol, or butanol at about 4 mg per ml at room temperature (solutions 1-3). Free base bromocriptine was dissolved in separate reaction vessels in either methanol, ethanol, or butanol at about 12 mg per 5-30 ml (solutions 4-6). The like organic solutions of citric acid and of bromocriptine (i.e., ethanol-ethanol, methanol-methanol, butanol-butanol) were then mixed in an equi-mole amount of bromocriptine and citrate. The three resulting solutions were stirred for about 2-24 hours on low heat (about 40 C) until the solvent evaporated to dryness. The resulting solid product in each reaction vessel contains bromocriptine citrate.
example 2
y of Bromocriptine Citrate Relative to Bromocriptine Mesylate
[0052]Solid samples of equal amounts of bromocriptine mesylate and bromocriptine citrate added, under various pH conditions, to equal volumes of water or water / organic solutions in different vessels and the dissolution of the bromocriptine samples (aqueous solubility) was assessed over time. Bromocriptine citrate was found to dissolve much more quickly and with significantly greater solubility (increased mg of bromocriptine dissolved per ml of water in the citrate vs mesylate salt form) compared to bromocriptine mesylate.
example 3
of Bromocriptine Citrate Relative to Bromocriptine Mesylate
[0053]Pharmaceutical preparations of bromocriptine mesylate and bromocriptine citrate are exposed to atmospheric conditions (40° C. and 70% relative humidity) and the degradation of the bromocriptine is assessed over time. The degradation of the bromocriptine from the citrate salt compound (bromocriptine citrate) is found to be substantially less than the degradation of the bromocriptine from the mesylate salt compound (bromocriptine mesylate) over a three-month period
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| total weight | aaaaa | aaaaa |
| total weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com