Cyclic galanin analogs and uses thereof
A cyclic analog, galanin technology, applied in the field of medicine and pharmacy, can solve the problem of reduced agonist specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0294] Example 1: Synthetic lambulfine - stabilized glypropanole analog (wool sulfur-glyproprine)
[0295] A glyproprine variant containing woolliternine is prepared according to the established method, for example, as described in the following literature: Kluskens 2005Post-Translational Modification of the Dehydrats of the lantibiotic nisin.biochemistry 44,12827 12834; Kluskens2009angiotensin- (1-7) with thioether-bridge: analogue.j.pharmacol.exper.j.pharmacol.exper.ther.328,849-854; rink 2007c Nisc, The Cyclase Offe Lantibiotic Nisin .,.
[0296] Briefly, lactococcus lactis includes two plasmid systems. The first plasmold coded antibiotic niobin (NISIN) preopeptide MSTKDFNLDLVSVSKDSGASPR, and its C-terminus and the objective in (meth) wool thikine-glyproprine precursor peptide fusion The above peptide contains serine / threonine at position [i], and has a cysteine at position [i + 3], [i + 4] or [I + 5]. Plasmids encoding a fusion peptide comprising a NiS precondition sequen...
Embodiment 2
[0299] Example 2: Activity of Wool Sulfide - Stabilized Glycopropeptide (Wool Sulfur - Glyceride)
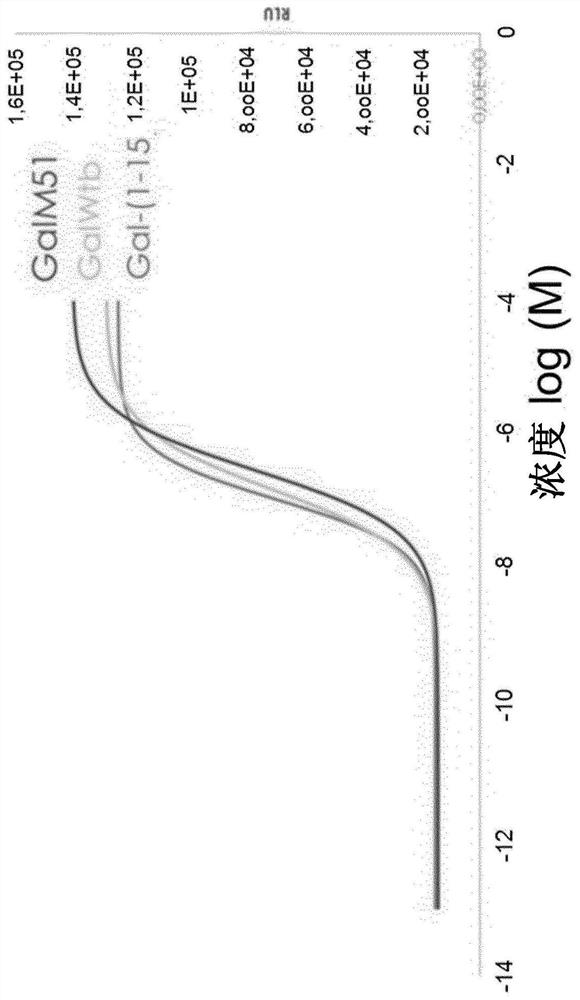
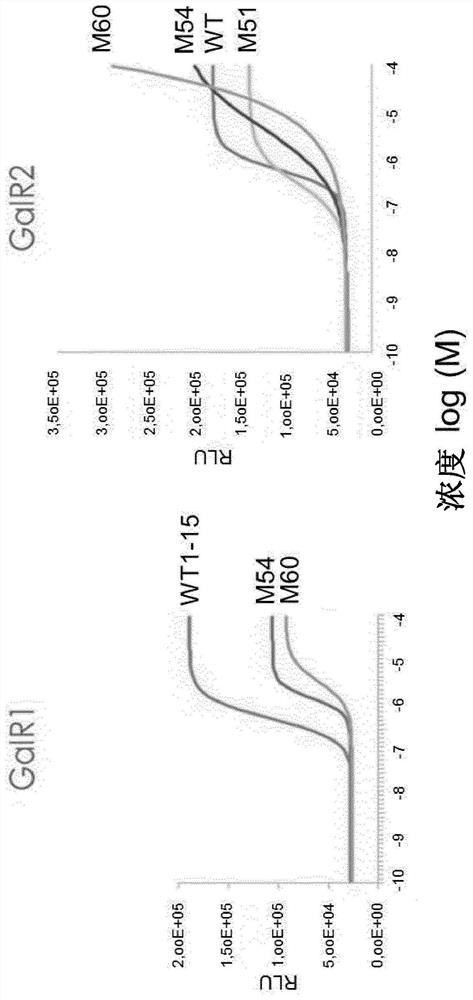
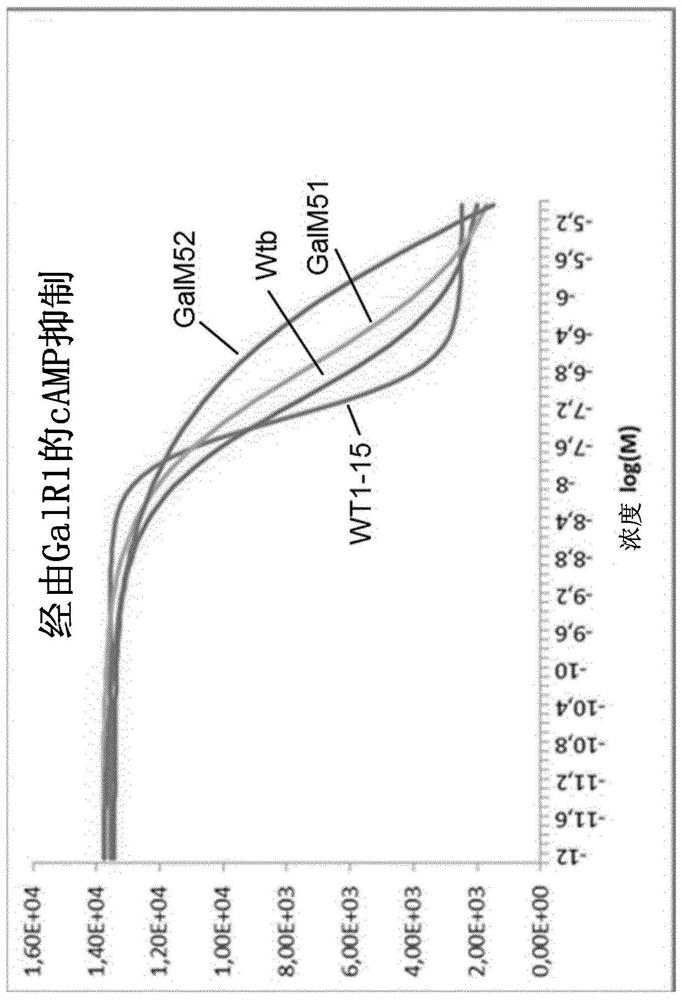
[0300] (Wool sulfur) glyprotinin biologically active was determined in the CHO-K1 cell line expressing GALR1 or GALR2 receptor. Active measurements were carried out using commercial kits for assaying inhibitory protein recruits (GALR1, GALR2) and CAMP synthesis inhibition (GALR1) for assaying inhibitory proteins. In addition, the HEK293 cell line expressing the GALR2 receptor has been used to measuring calcium flow (GALR2).
[0301]
[0302]
[0303]
[0304] result
[0305] The results are summarized in Table 1. The numerical value refers to an EC50 of an analog to which the expression observed with a linear glycetin (1-15) set to the value of 1.0. ND refers to "not determined".
[0306] Surprisingly, it is discovered that (meth) wool hydrazine is introduced into the C-terminal of glyproprine so that the direction towards the GALR2 tend to migrate. Specifically, GALM50B, GA...
Embodiment 3
[0310] Chemical synthetic lanamine thiramine thiramine thiopartide is extruded by base-assisted sulfur sulfur to obtain glyproprine.
[0311] Ordering two peptides in Pepscan as raw materials:
[0312] 1) SYM-4178: H-Qwnlnaagyllgpcavc-oh
[0313] The peptide is related to GALM87. However, although biosynthetic Galm87 may contain D, L wool thiternine, chemical synthesis results in an or more isomer. Interestingly, one of the isomers containing wool thionic isomers (Table 2) have high activity.
[0314] 2) SYM-4179: H-QWTLNAACYLLC-OH
[0315] The peptide is related to GALM82 (Table 1). However, although biosynthetic Galm82 may contain D, L wool thiosine, chemical synthesis results in one or more isomers. More interesting is that one of these 4179b (Table 2) seems to have high activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com