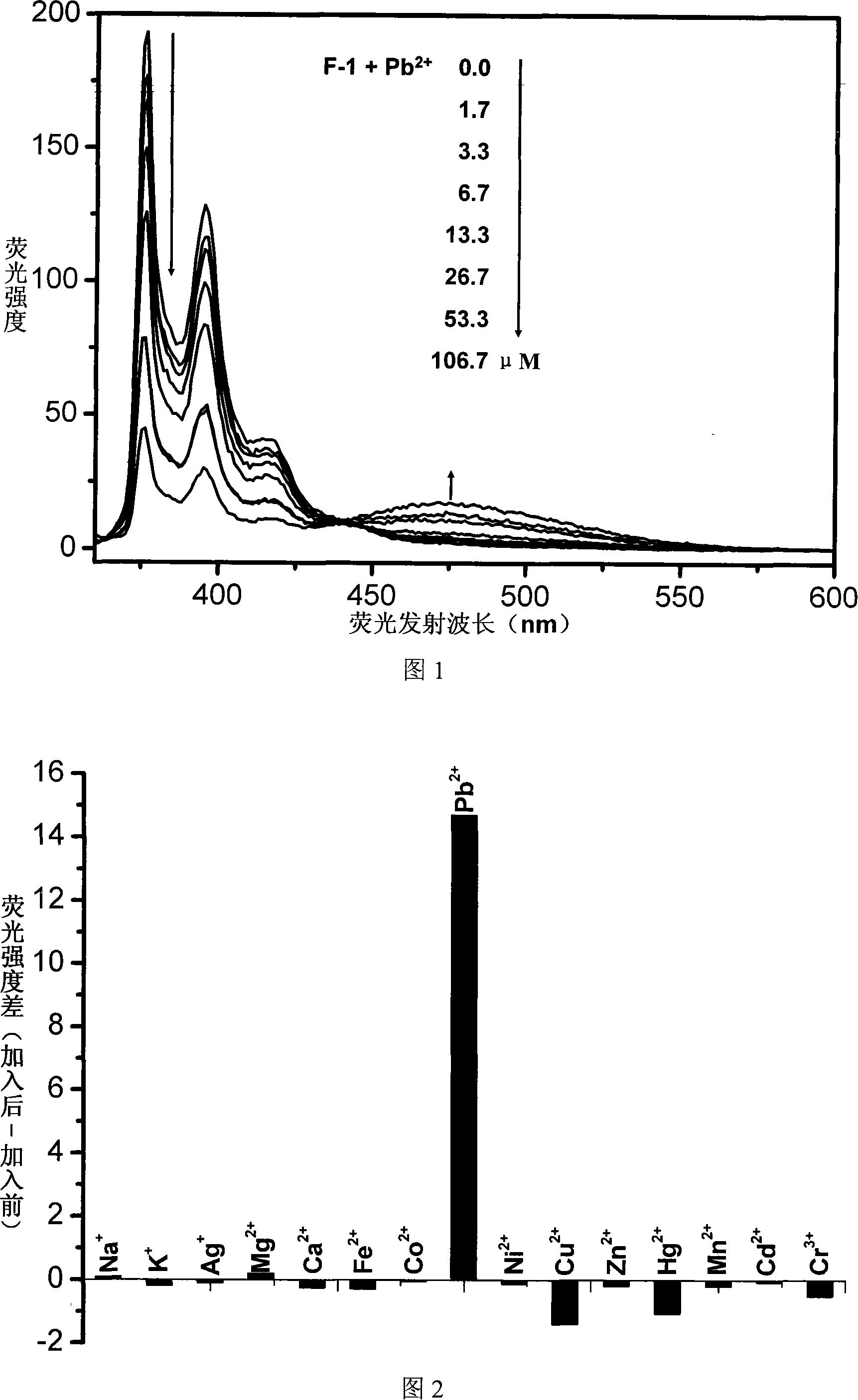
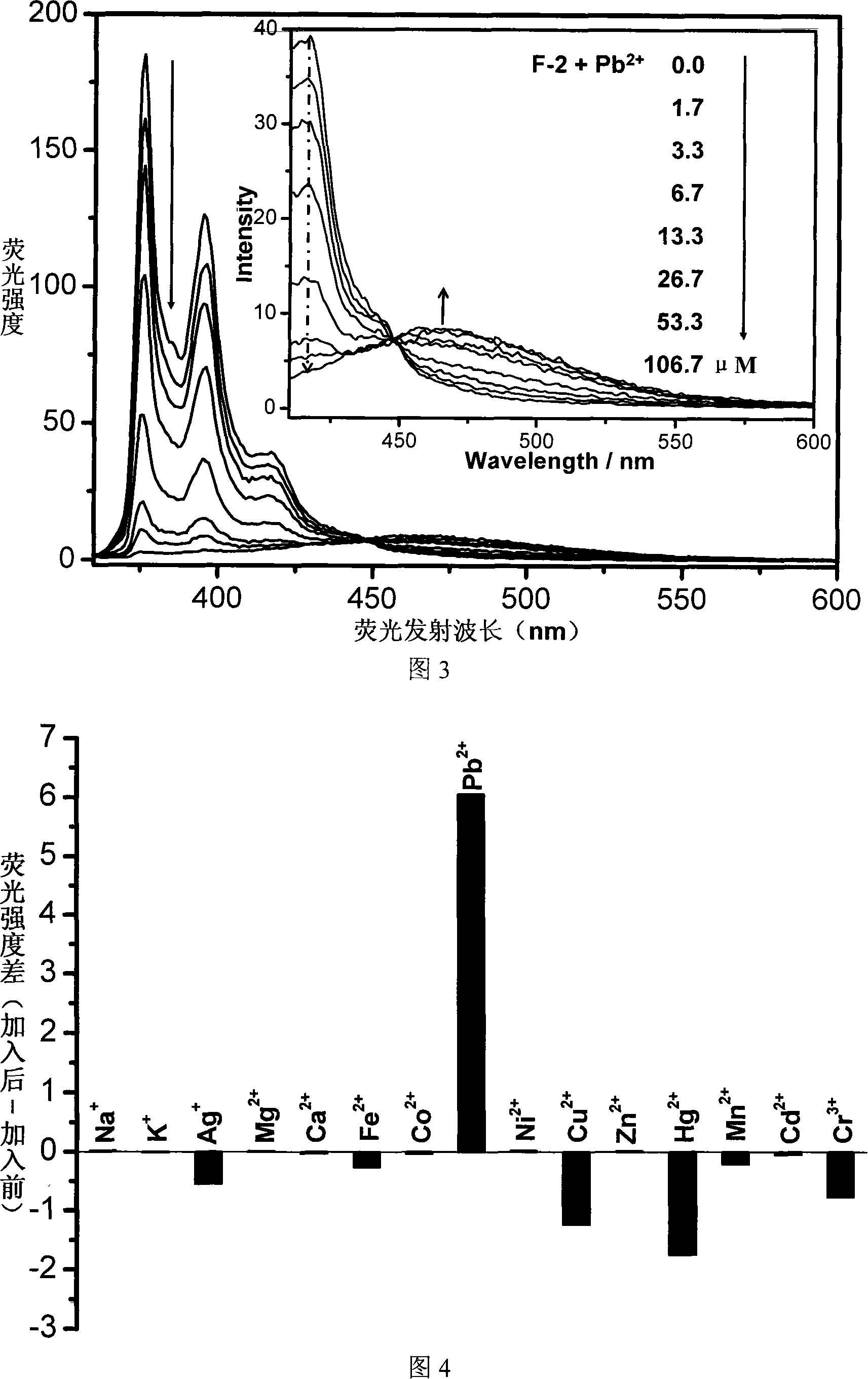
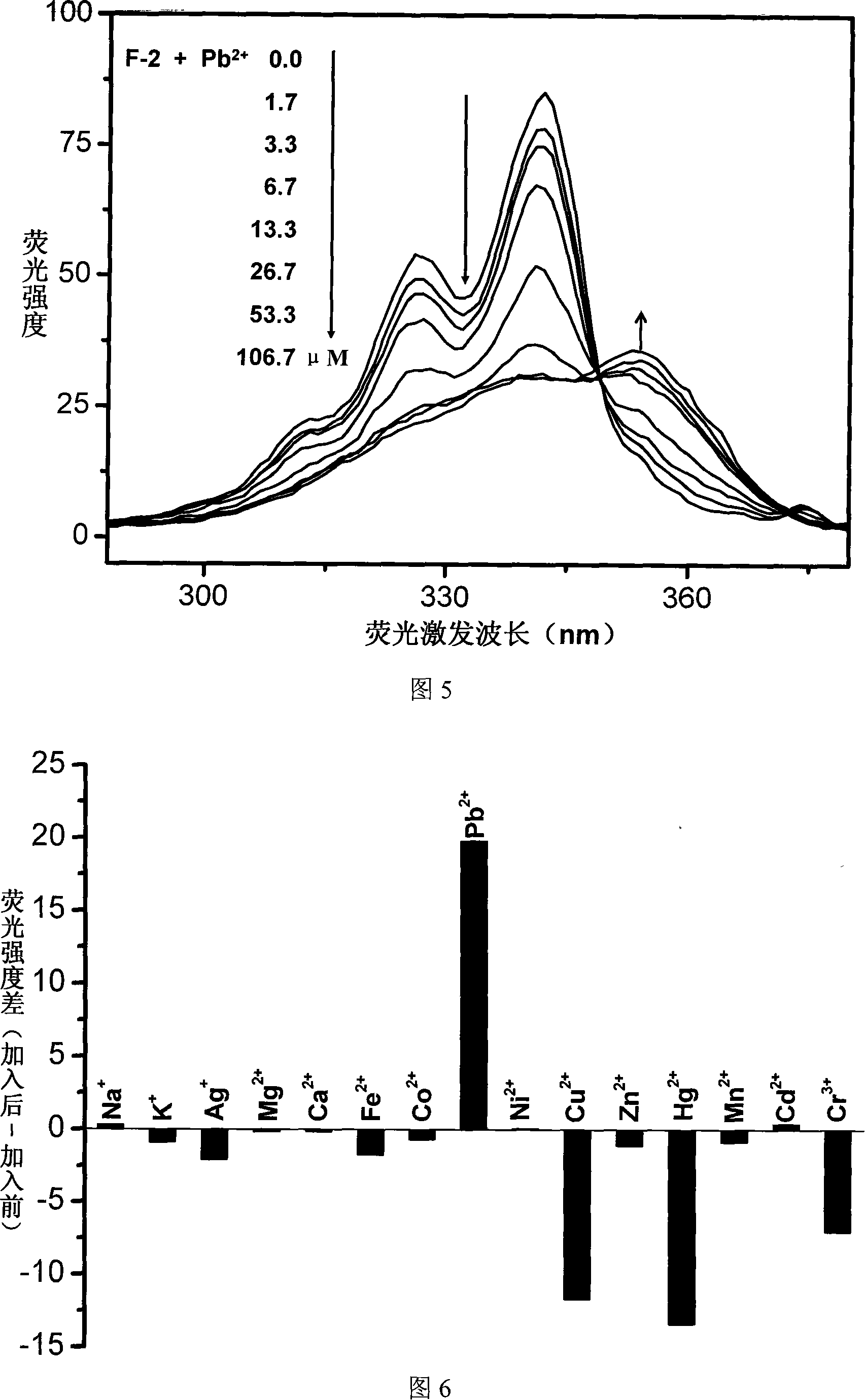
Fluorescent ion probe and its application in ion detecting
A technology of fluorescent ions and probes, applied in the field of fluorescent ion probes, can solve problems such as very different selectivity or sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
[0066] F-1 in this example includes L-configuration, D-configuration and racemate D, L-mixed configuration, the method is the same, the phenomenon is the same, and the yield is also the same. The following embodiments 2-8 also include three configurations, and the descriptions of the following several embodiments are omitted. The amino acids used in the examples all include L-configuration, D-configuration and racemate D, L-configuration, and descriptions are also omitted for the following examples.
[0067] Add 5.0 mL of anhydrous methanol to a round bottom flask, place it in an ice-salt bath to cool for 5 minutes, and slowly add 0.70 mL of thionyl chloride dropwise. After the system continued to stir for 10 min, 1.0 g of aspartic acid was added at one time, and the mixed system was stirred at room temperature for 2 h. Then put the reaction system in a water bath at 70-80°C to reflux for 30 minutes, evaporate part of the methanol, add 40 mL of anhydrous diethyl e...
Embodiment 2
[0076]
[0077] Dissolve 0.40g of pyrenebutyric acid in 40mL of benzene, add 1.0mL of thionyl chloride, heat and reflux and stir for 7h, after the reaction is complete, distill off the organic solvent benzene under reduced pressure to obtain a yellow crude product, which is then recrystallized with n-hexane for 3 Second-rate. A yellow-brown solid was precipitated from the n-hexane crystallization liquid, which was 0.27 g of pyrenebutyryl chloride, and the yield was 62%.
[0078] The preparation method of dimethyl glutamate hydrochloride and dimethyl glutamate (B) is similar to the preparation method of dimethyl aspartate hydrochloride and dimethyl aspartate in Example 1, Just change aspartic acid into glutamic acid, the molar ratio of reactant is constant, reaction condition and step, process are identical, can obtain white solid dimethyl glutamate hydrochloride 1.1g, productive rate 77%, Its structural formula is:
[0079]
[0080] That 1 H-NMR spectrum (500MHz, D 2...
Embodiment 3
[0086]
[0087] The preparation method of tryptophan methyl ester hydrochloride and tryptophan methyl ester (C) is the same as the preparation method of aspartic acid dimethyl ester hydrochloride and aspartic acid dimethyl ester in embodiment 1-1, Exactly aspartic acid is replaced by tryptophan, the molar ratio of the reactants is constant, the reaction conditions are similar to the steps, and the process is similar, so that 1.0 g of micropink solid powder tryptophan methyl ester hydrochloride can be obtained, and the yield is 80%, its structural formula is:
[0088]
[0089] That 1 H-NMR spectrum (500MHz, D 2O) Chemical shifts and corresponding proton type assignments are: 7.576-7.134 (m, 5H), 4.402 (m, 1H), 3.747 (s, 3H), 3.483-3.390ppm (m, 2H).
[0090] Further, 0.17 g of tryptophan methyl ester was obtained as a pale yellow oil, with a yield of 67%. Likewise, the resulting tryptophan methyl ester is unstable and should be used immediately.
[0091] The synthesis ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com