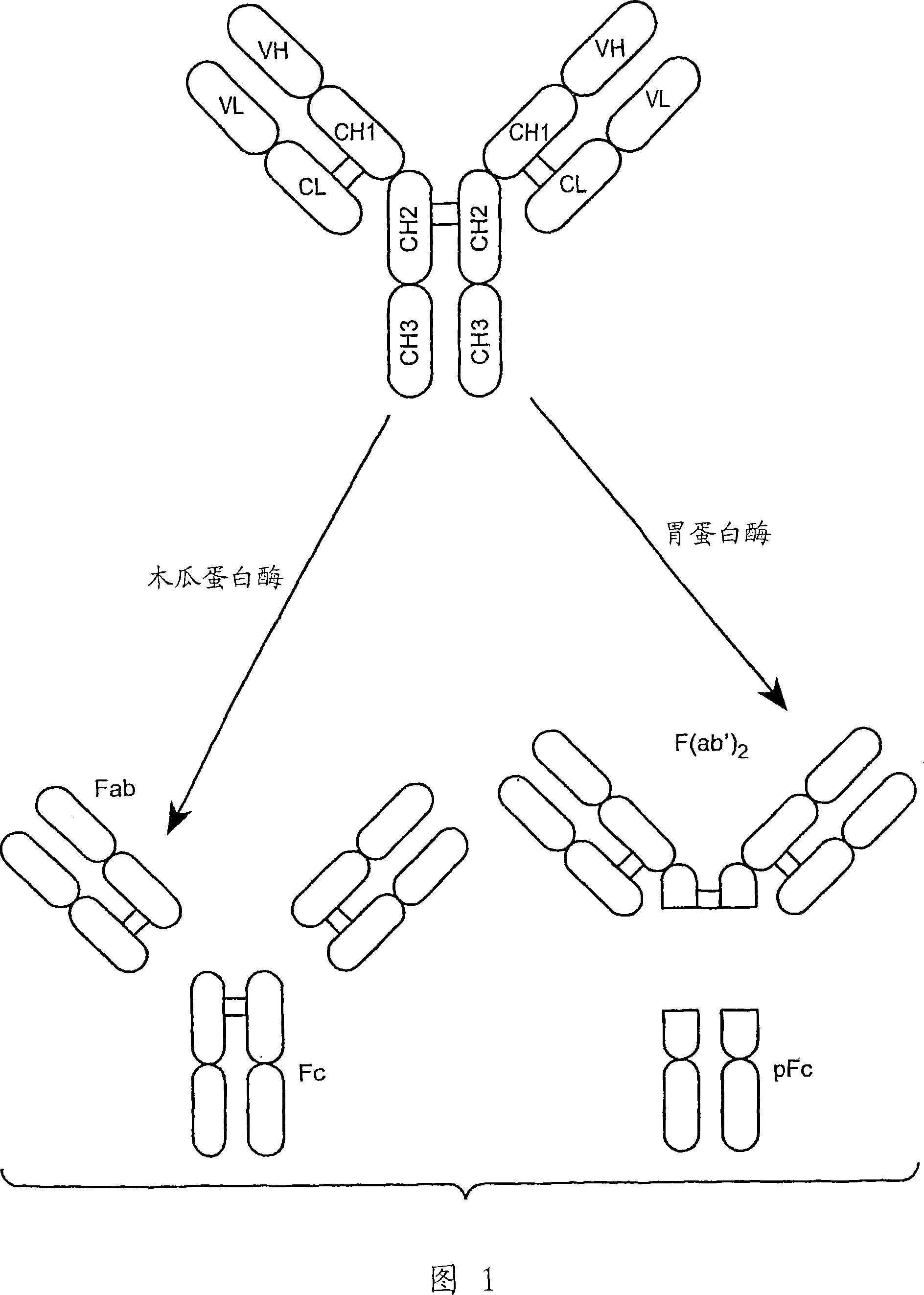
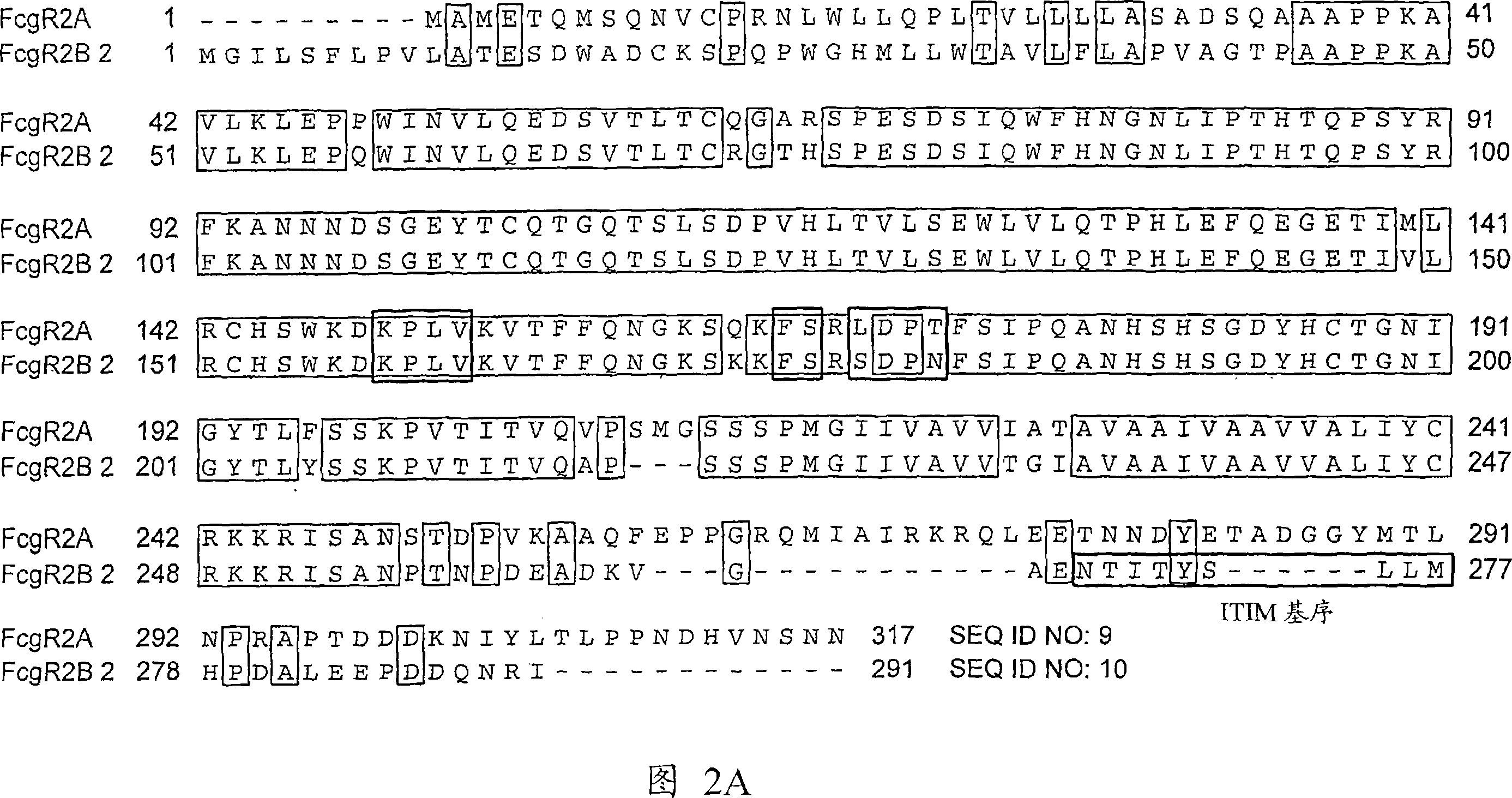
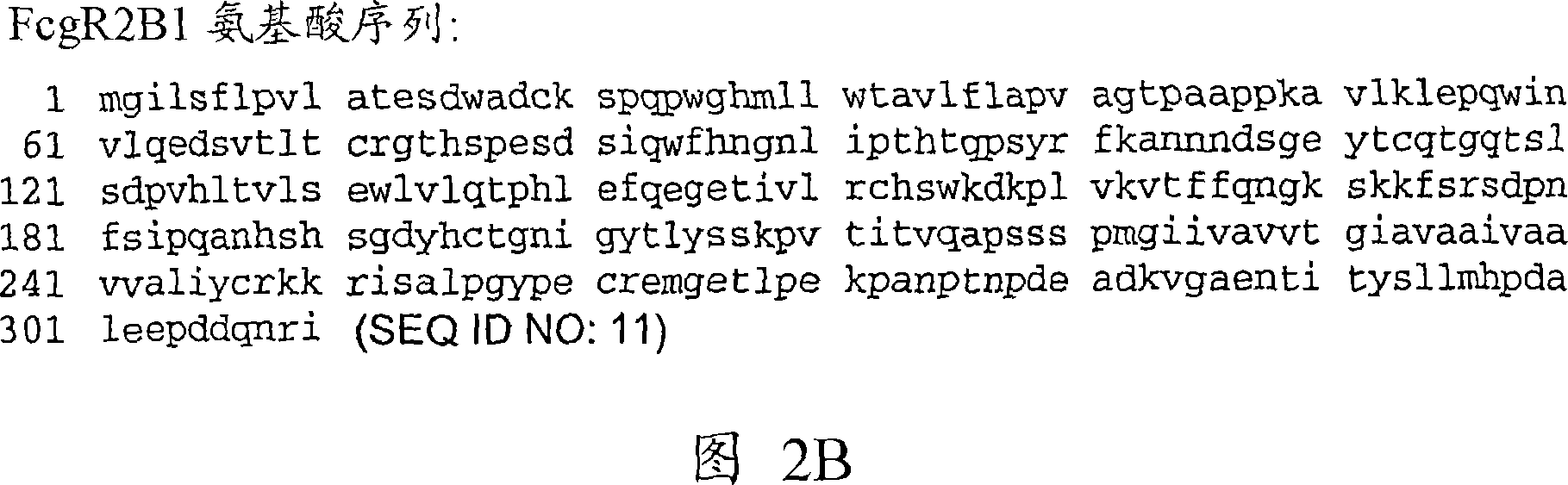
Anti-Fc-gamma RIIB receptor antibody and uses therefor
An antibody and receptor technology, applied in the direction of antibodies, anti-receptors/cell surface antigens/cell surface determinants, immunoglobulins, anti-inflammatory agents, etc., can solve problems such as functional differences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 10
[0288] Example 1.0 Materials and methods
[0289] 1.1 Materials
[0290] Reverse transcription PCR using Perkin Elmer Life Sciences GeneAmp TM conduct.
[0291] pGEX-4T2 plasmid, protein A column and reagents, and protein G FcyRIII: columns and reagents were obtained from Amersham Pharmacia Biotech. Ni-NTA columns and reagents were from Qiagen, Valencia, CA. Centriprep-30 concentrators were from Millipore, Bedford, MA. SDS-polyacrylamide gels and polyvinylidene fluoride membranes were obtained from NOVEX, San Diego, CA. FuGENE(R) 6 was obtained from Roche.
[0292] Encodes human FcγRIIA (CD32A; His 131 allotype), FcγRIIB (CD32B), and FcγRIIIA (CD16A; Val 158 allotype) and the glucose-6-phosphate isomerase (GPI) isoform of FcyRIIB and the cDNAs for the extracellular and transmembrane domains of FcyRIIA were provided by Dr. J. Ravetch (Rockefeller University, New York). FcγRIIA-Arg 131 Allotypes and FcγRIIIA-Phe 158 Allotypes were generated by site-directed mutagenesis...
Embodiment 2
[0317] Example 2.0 Properties of anti-FcγRIIB antibodies
[0318] 2.1 Materials
[0319] Anti-FcεRI MAb, 22E7 Mab binds FcεRI, with or without IgE bound to the receptor. 22E7 Mab was purified from the Hoffman-LaRoche cell line IGE4R: 22E7.2D2.1D11 (Risek, F., et al., 1991, J. Biol. Chem. 266: 11245-11251). Hoffman-LaRoche cells expressing 22E7 Mab were cultured in Iscove's modified Dulbecco's medium containing 10x FBS, 1x Pen-Strep, and 1x glutamine. 22E7 MAb was purified by protein A and protein G chromatography. 22E7 extracts were pooled and affinity for FcεRI was verified.
[0320] 2.2 RBL cell lines
[0321] The RBL48 cell line, derived from the parental rat mast cell line RBL-2H3 (ATCC #CRL-2256), expresses the alpha subunit of the high-affinity human IgE receptor (FcεRI) (Gilfillian A.M. et al., 1992, Immunology 149:2445- 2451). The cDNA clone (Muta T., et al.) of the human FcγRIIB1 full-length α subunit has been subcloned into a puromycin-selectable expression vec...
Embodiment 30
[0326] Example 3.0 Generation of bispecific antibodies
[0327] This example describes the construction and purification of bispecific antibodies that have a variant hinge region that lacks disulfide bond-forming cysteine residues ("hingeless"). The construction of bispecific antibodies with wild-type hinge sequences is also described; these antibodies can be used to assess the efficiency of obtaining various types of antibody complexes.
[0328] 3.1 Construction of expression vector
[0329] All plasmids used to express full-length antibodies are based on a separate cistron system (Simmons et al., 2002, J. Immunol. Methods 263:133-147; Simmons et al., US Patent 5,840,523), which rely on a separate phoA Promoter (AP) (Kikuchi et al., 1981, Nucleic Acids Res.9: 5671-5678) to transcribe heavy and light chains, followed by trp Shine-Dalgamo sequence to initiate translation (Yanofsky et al., 1981, Nucleic Acids Res 9: 6647-6668; Chang et al., 1987, Gene 55: 189-196). In addit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com