Composition comprising the extract of actinidia arguta and related species for the prevention and treatment of allergic disease and non-allergic inflammatory disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Hardy Kiwifruit Extract
1-1. Preparation of Water Extract of Hardy Kiwifruit
[0128] 100 g of dried hardy kiwifruit and dried stem of hardy kiwifruit (Actinidia arguta), dried fruit of A. kolomikta and A. polygama purchased from Kyung-dong Market located in Seoul was crushed, mixed with 1 L of distilled water and subjected to reflux extraction for 3 hrs at 90˜95° C. with three times and the extract was filtered with filter paper, concentrated using by rotary evaporator (N-1000, Eyela Co. Japan) at 55˜65° C. under reduced pressure and dried with freezing dryer to obtain 15.6 g of dried fruit extract, 10.4 g of dried stem extract of kiwifruit (Actinidia arguta), 16.2 g and 17.0 g of dried fruit extract of A. kolomikta, and A. polygama respectively. The dried powder was dissolved in distilled water (100 mg / ml).
1-2. Preparation of Water-alcohol Soluble Extract of Hardy Kiwifruit
[0129] Except using various mix ratio of water-alcohol solvent mixture such as 30%, 50%, and...
example 2
Preparation of Polar Solvent and Non-polar Solvent Soluble Hardy Kiwifruit Extract
[0130] The water extract prepared in Example 1-1 was subject to fractionation by following procedure.
2-1. Preparation of Chloroform Soluble Fraction
[0131] 50 ml of distilled water was added to 5 g of hardy kiwifruit extract obtained in Example 1-1. 50 ml of chloroform was added thereto in separatory funnel, shaken vigorously to divide into chloroform soluble layer and water soluble layer.
2-2. Preparation of Ethyl Acetate Soluble Fraction
[0132] Above water soluble layer obtained in Example 1-1 was mixed with 50 ml of ethyl acetate and then divided into ethyl acetate soluble layer and water soluble layer.
[0133] Above chloroform soluble layer, ethyl acetate soluble layer and water layer were concentrated by rotary evaporator, dried with freeze dryer to obtain 0.34 g of chloroform soluble fraction, 0.05 g of ethyl acetate soluble fraction and 4.61 g of water fraction powders respectively.
example 3
Fractionation of Hardy Kiwifruit Extract by Silica Gel Column Chromatography
[0134] 2,784 mg of ethyl acetate soluble fraction in Example 2-2 was further subjected to silica gel column chromatography (Daiso gel IR-60-W-40:63 mm). The developing solvent was started with chloroform:methanol:water ([1] 90:11:1, [2] 60:10:1, [3] 60:20:2) solvent mixture and ended with methanol[4] with eluting speed of 300 ml / hr to obtain four sub-fractions([1] 2,381 mg, [2] 135 mg, [3] 148 mg, [4] 98 mg).
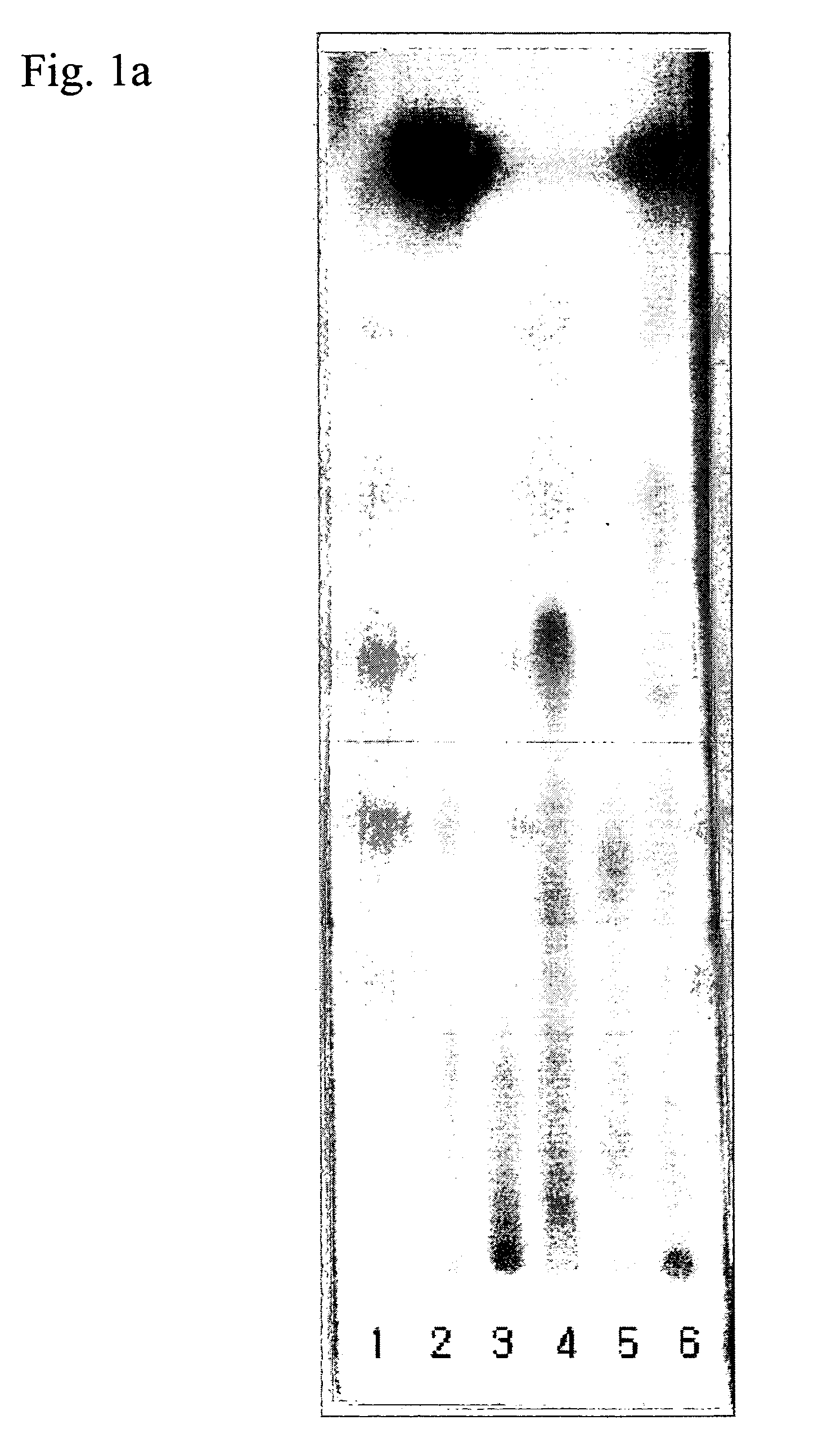
[0135] Above water extract, ethyl acetate soluble fraction and four sub-fractions were subjected to TLC (TLC plate: Merck Co. Ltd., Developing solvent; chloroform:methanol:water=9:5:1) and the results were shown in FIG. 1a. As shown in FIG. 1a, lane 1 is water extract, lane 2 is ethyl acetate soluble fraction, lane 3 is [4] sub-fraction, lane 4 is [3] sub-fraction, lane 5 is [2] sub-fraction and lane 6 is [1] sub-fraction.
[0136] Above [1] and [2] sub-fractions were subjected to 2D-TLC using chloroform...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com