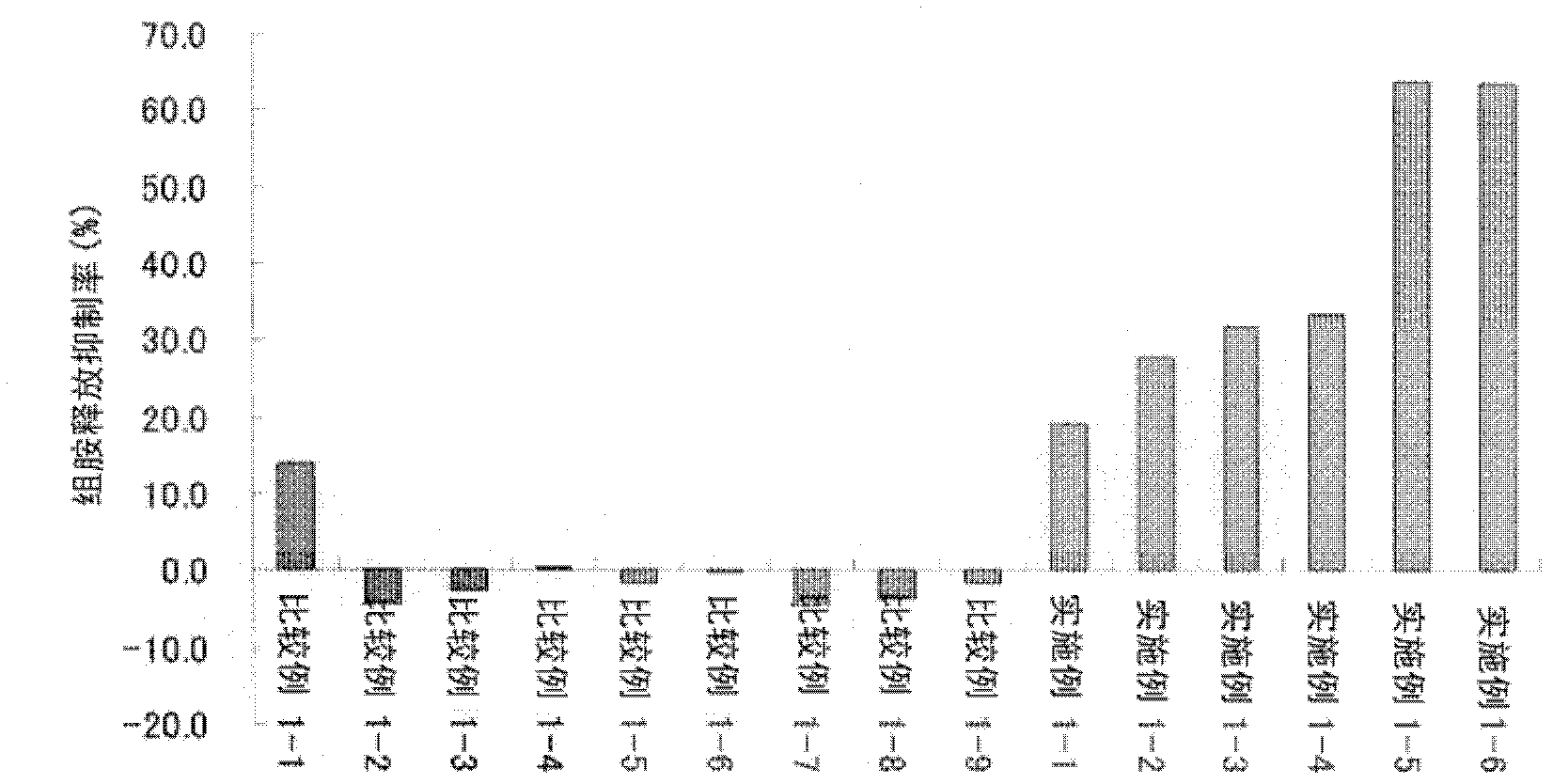
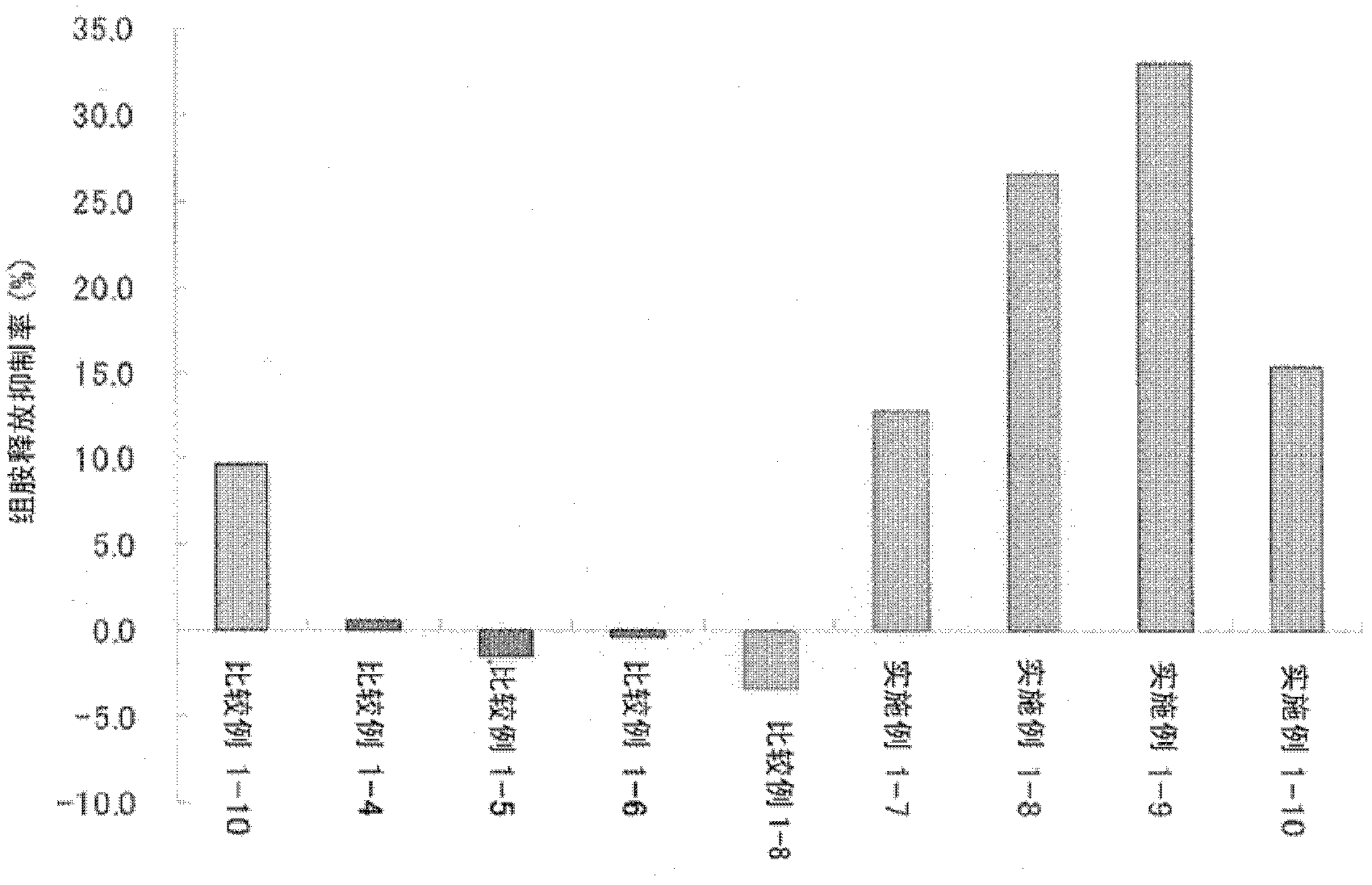
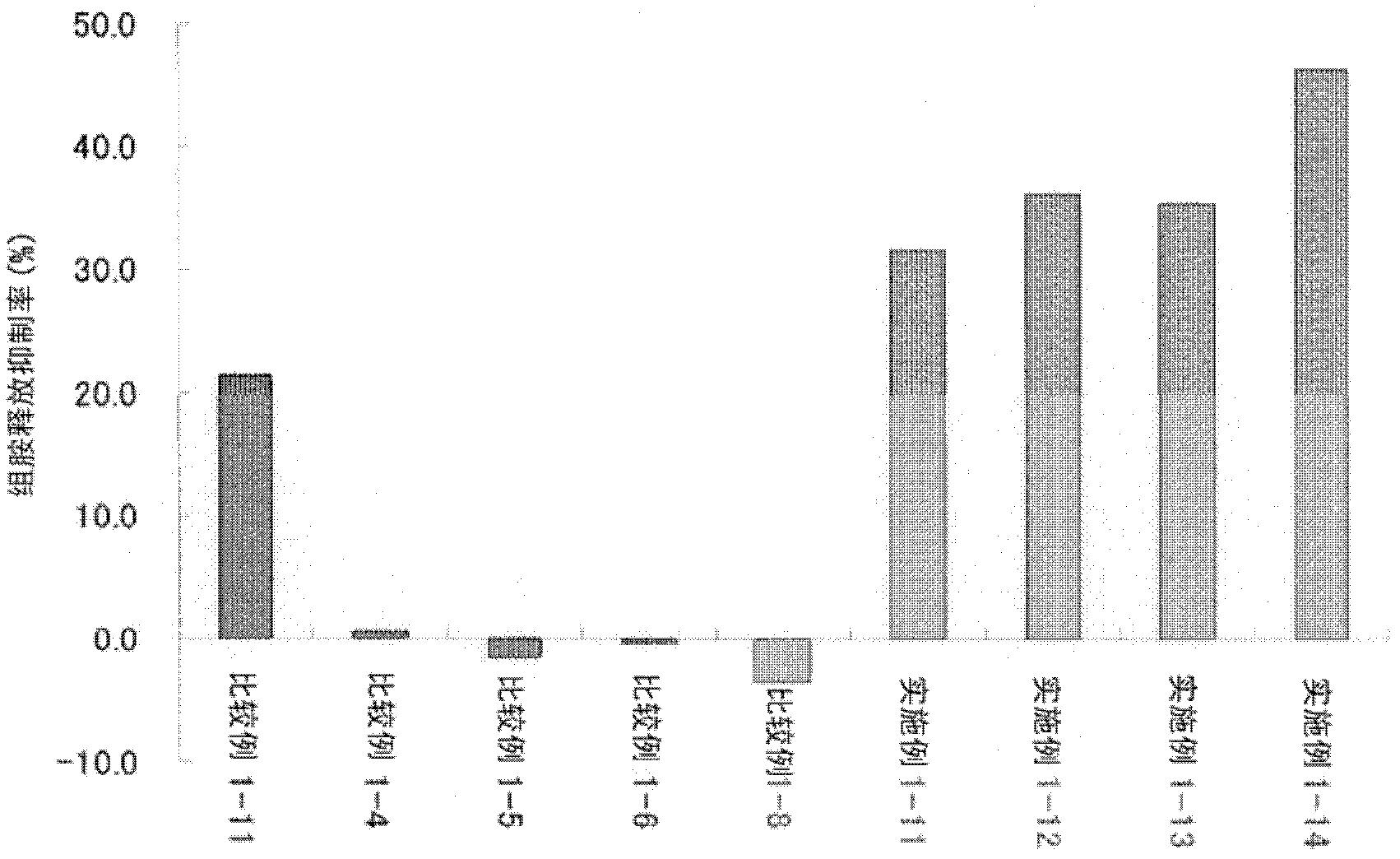
Ophthalmic composition
A composition, ophthalmic technology, applied in the directions of drug combination, active ingredients of hydroxyl compounds, drug delivery, etc., can solve the problem of unknown histamine, unpredictable effects of histamine release, and no discussion on the effects of silicone hydrogel lenses, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0266]
[0267] #1 MPC polymer is the same polymer used in Table 1.
[0268] The prescription of the ophthalmic composition of the above-mentioned formulation example 1 was prepared according to a conventional method, and 15 mL was filled into a 15-mL PET container, and an eye drop and an eye drop for SHCL were prepared respectively.
preparation example 2
[0270]
[0271]
[0272] #1 MPC polymer is the same polymer used in Table 1.
[0273] The ophthalmic composition prescription of the above-mentioned formulation example 2 was prepared according to a conventional method, and 10 mL was filled into a 10 mL capacity PET container to prepare eye drops, contact lens filling solutions, eye drops for SHCL, and contact lens filling solutions for SHCL, respectively. In addition, instead of the above-mentioned container, 500 mL was filled into a 500 mL-capacity PET container, and an eye wash and an eye wash for SHCL were produced, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com