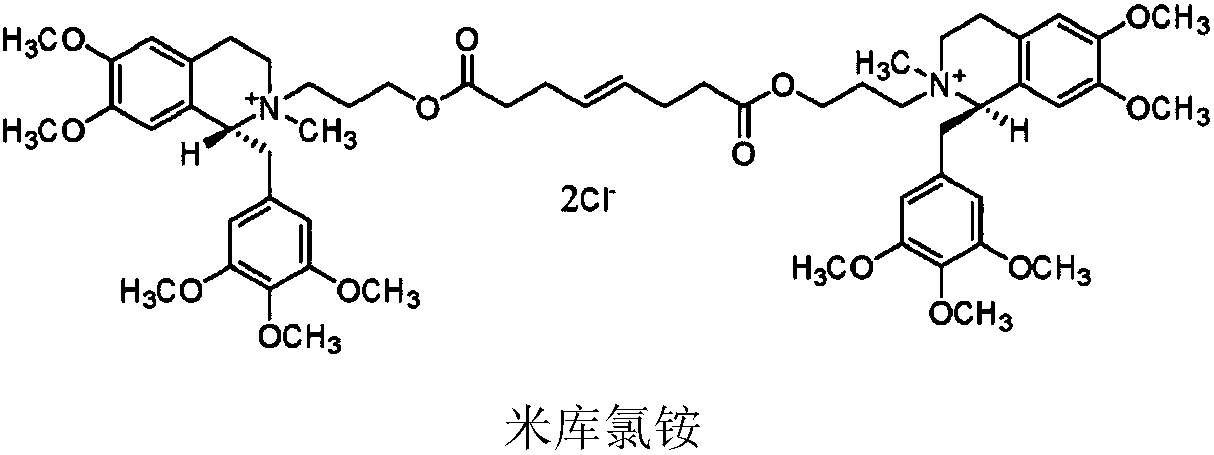
Non-depolarizing muscle relaxant composition as well as preparation method and application thereof
A composition and compound technology, applied in the directions of drug combination, pharmaceutical formulation, muscular system diseases, etc., can solve problems such as affecting the cardiovascular system, allergies, skin flushing, etc., achieving good storage stability, small release effect of histamine, and effective fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
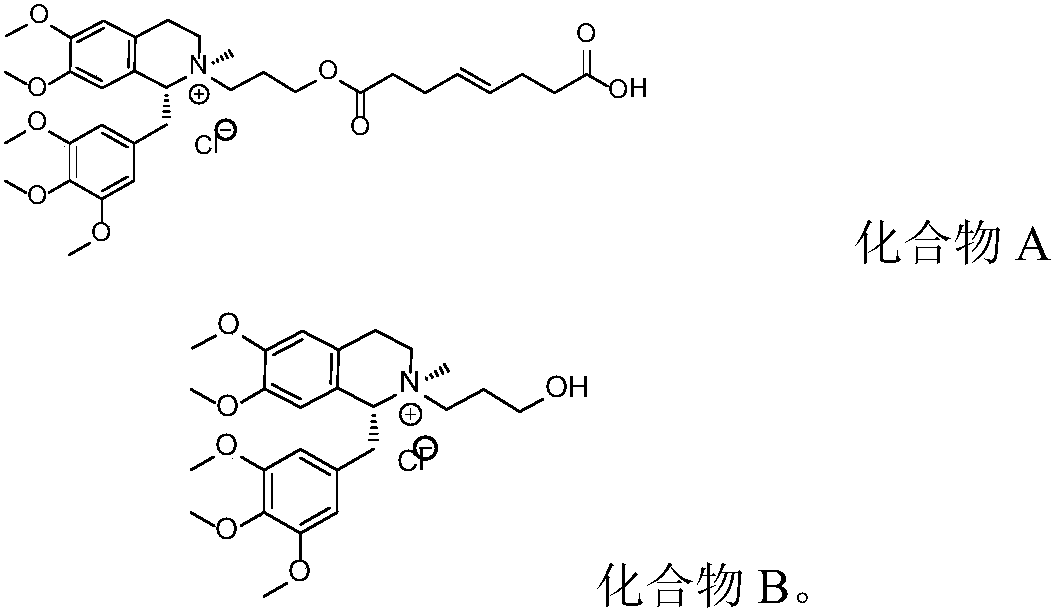
[0052] Weigh 100.0g (R)-5'-methoxylaudansu, 63.0g 3-chloropropanol, 115.0g sodium iodide, 10.0g anhydrous sodium carbonate, add solvent acetone 1500mL, reflux for 24h. After the reaction is complete, stop heating, cool down and filter, concentrate the filtrate to dryness, add purified water to dissolve, then add dichloromethane to extract, separate liquid, pass the water layer through an ion exchange resin column, collect the liquid, add sodium chloride to saturate, and dichloromethane extract , concentrated to dryness to give 6,7-dimethoxy-2-(3-hydroxypropyl)-2-methyl-1-[(3,4,5-trimethoxyphenyl)methyl]-1 ,2,3,4-tetrahydroisoquinoline chloride. The purity determined by HPLC was 99.72%.
preparation example 2
[0054] Weigh 100.0g (R)-5'-methoxylaudansu, 63.0g 3-chloropropanol, 115.0g sodium iodide, 10.0g anhydrous sodium carbonate, add solvent acetone 1000mL, reflux for 20h. After the reaction is complete, stop heating, cool down and filter, concentrate the filtrate to dryness, add purified water to dissolve, then add chloroform to extract, separate liquid, pass the water layer through an ion exchange resin column, collect the liquid, add sodium chloride to saturate, extract with dichloromethane, and concentrate to dry to obtain 6,7-dimethoxy-2-(3-hydroxypropyl)-2-methyl-1-[(3,4,5-trimethoxyphenyl)methyl]-1,2 ,3,4-tetrahydroisoquinoline chloride. The purity determined by HPLC was 99.72%.
preparation example 3
[0056] Weigh 100.0g (R)-5'-methoxylaudansu, 63.0g 3-chloropropanol, 115.0g sodium iodide, 10.0g anhydrous sodium carbonate, add solvent acetone 800mL, reflux for 18h. After the reaction is complete, stop heating, cool down and filter, concentrate the filtrate to dryness, add purified water to dissolve, add 1,2-dichloroethane to extract, separate liquid, pass the water layer through an ion exchange resin column, collect the liquid, add sodium chloride to saturate , extracted with dichloromethane, and concentrated to dryness to give 6,7-dimethoxy-2-(3-hydroxypropyl)-2-methyl-1-[(3,4,5-trimethoxyphenyl) Methyl]-1,2,3,4-tetrahydroisoquinoline chloride. The purity was determined to be 99.75% by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com