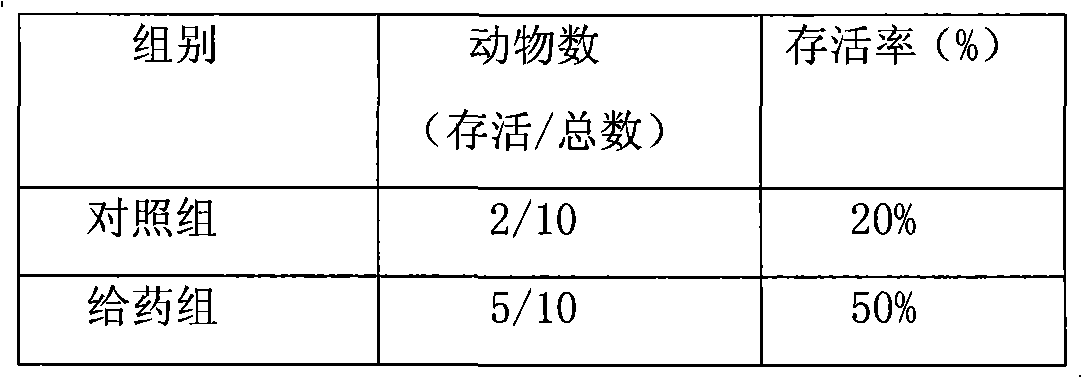
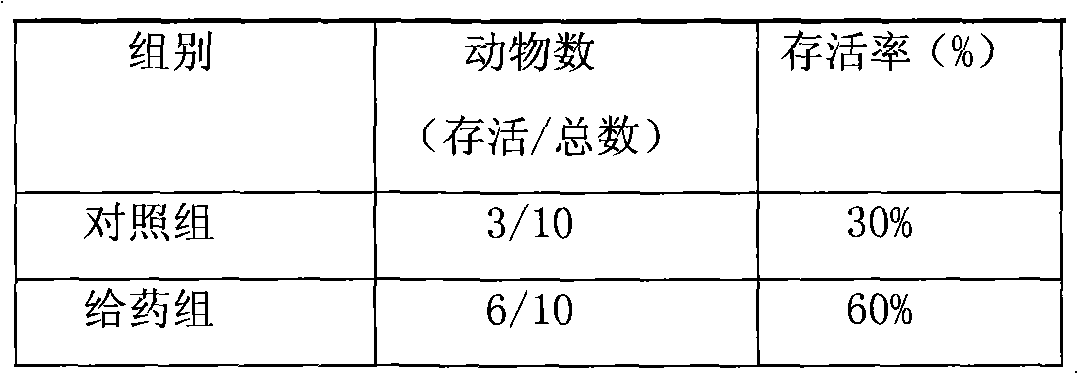
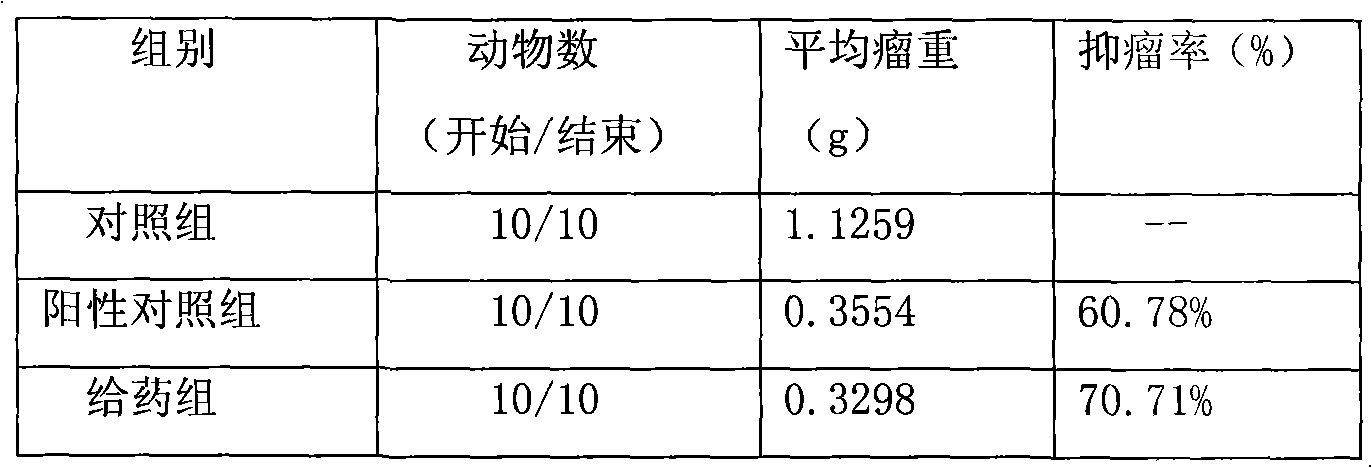
Anti-cancer drug preparation taking Solutol HS 15 as solubilizer
A technology for anticancer drugs and preparations, applied in the field of medicine, can solve the problems of patient use risks, inconvenient clinical medication, interference with p-glycoprotein expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] prescription:
[0024] Paclitaxel: 2.0g
[0025] Polyethylene glycol-12-hydroxystearate: 200g
[0026] Glucose: 100g
[0027] Mannitol: 80g
[0028] PBS solution (pH 5.8): 1000ml
[0029] Method: Dissolve 2.0g paclitaxel in 200g Solutol HS 15 at a certain temperature, stir until clear to obtain solution 1, dissolve 100g glucose and 80g mannitol in an appropriate amount of PBS solution, stir until clear to obtain solution 2; 1. Mix solution 2, depyrogenate with activated carbon, adjust the volume of PBS solution to 1000ml, adjust the pH value to 5.5 with 0.1M NaOH or 0.1M HCL, filter and sterilize, aliquot and freeze-dry.
Embodiment 2
[0031] prescription:
[0032] Paclitaxel: 3.0g
[0033] Polyethylene glycol-12-hydroxystearate: 300g
[0034] Sucrose: 200g
[0035] Mannitol: 50g
[0036] Water for injection: up to 1000ml
[0037] Method: Dissolve 3.0g paclitaxel in 300g Solutol HS 15 at a certain temperature and stir until clear to obtain solution 1. Dissolve 200g sucrose and 50g mannitol in an appropriate amount of water for injection and stir until clear to obtain solution 2. 1. Mix solution 2, depyrogenate with activated carbon, adjust the volume to 1000ml with water for injection, adjust the pH value to 6.5 with 0.1M NaOH or 0.1M HCL, filter and sterilize, aliquot and freeze-dry.
Embodiment 3
[0039] prescription:
[0040] Paclitaxel: 4.0g
[0041] Polyethylene glycol-12-hydroxystearate: 400g
[0042] Trehalose: 200g
[0043] Lactose: 100g
[0044] VC: 0.5g
[0045] PBS solution (pH 5.0): up to 1000ml
[0046] Method: Dissolve 4.0g paclitaxel in 400g Solutol HS 15 at a certain temperature, stir until clear to obtain solution 1, dissolve 200g trehalose, 100g mannitol, and 0.5g VC in an appropriate amount of PBS solution, stir until clear to obtain a solution 2. Mix solution 1 and solution 2 evenly, depyrogenate with activated carbon, adjust the volume of PBS solution to 1000ml, adjust the pH value to 4.5 with 0.1M NaOH or 0.1M HCL, filter and sterilize, aliquot and freeze-dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com