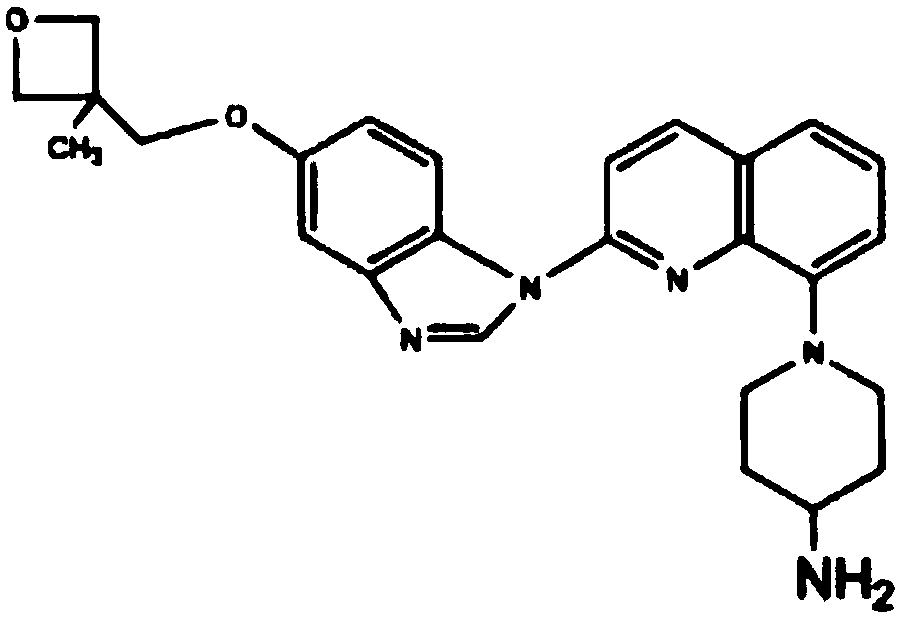
Pharmaceutical composition containing crenolanib and application of pharmaceutical composition
A technology of clalanib and its use, which is applied in the field of pharmaceutical compositions containing clalanib and its use, and can solve problems such as dose escalation and treatment end
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Preparation of Compounds of the Invention. General synthetic methods for preparing compounds of formula I are provided in, for example, U.S. Patent No. 5,990,146 (issued November 23, 1999) (Warner-Lambert Co.) and PCT Published Application No. WO 99 / 16755 (published April 8, 1999) (Merck & Co.), WO 01 / 40217 (published July 7, 2001) (Pfizer, Inc.), U.S. Patent Application Publication No. US 2005 / 0124599 (Pfizer, Inc.) and US Patent No. 7,183,414 (Pfizer, Inc.), in relevant part, is incorporated herein by reference.
[0058] Pharmaceutically acceptable salts such as hydrochloride, phosphate and lactate are prepared in a similar manner to the besylate salts and are well known to those of ordinary skill in the art. The following representative compounds of the invention are for illustrative purposes only and are by no means meant to limit the invention, including clalanid besylate, clalanide phosphate, clalanide lactate, clalanide hydrochloride, lemon Clairaninate, Clella...
Embodiment 1
[0060] Efficacy of combination therapy with clalanib besylate in patients with gastroesophageal junction (GEJ) adenocarcinoma with liver metastases: partial response according to RECIST 1.1 criteria.
[0061] A 53-year-old male was initially diagnosed with esophageal cancer in September 2017. Patients received palliative FOLFOX chemotherapy: a combination of a fluoropyrimidine (fluorouracil or 5-FU), a platinum compound (oxaliplatin) and leucovorin. Palliative chemotherapy started in late September 2017 and continued until January 2018. The patient was then given oral capecitabine for 5 months until June 2018 when the patient's cancer progressed. CT scans showed that the patient had progressive disease and the cancer had metastasized to the liver, upper stomach and lymph nodes.
[0062] For disease progression on first-line therapy, this patient was offered oral clalanib besylate (PDGFRβ inhibitor) with paclitaxel and ramucirumab in a second-line clinical trial of advanced es...
Embodiment 2
[0064] Efficacy of combined therapy of clalanib besylate in patients with stage IIIB gastric adenocarcinoma with metastasis to lymph nodes: Partial response was assessed according to RECIST 1.1 criteria.
[0065] A 68-year-old woman was diagnosed in September 2015 with stage IIIB metastatic cancer in the stomach and lymph nodes. She underwent partial gastrectomy and started adjuvant FOLFOX chemotherapy in October 2015. She remained on the FOLFOX regimen for 12 cycles until April 2016. Despite adjuvant chemotherapy, a CAT scan in October 2016 showed increased metastatic disease in the lymph nodes, which prompted palliative chemotherapy with fluorouracil. The patient continued to progress and was found to have metastatic disease to the gastrointestinal ligaments, lungs, and lymph nodes in October 2017.
[0066] Oral clalanib besylate (PDGFRβ inhibitor) was given to patients in combination with paclitaxel and ramucirumab in a second-line clinical trial (NCT03193918) of advanced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com