Application of axitinib and PX-478 in treatment of nasopharyngeal carcinoma
A PX-478, 1. PX-478 technology, used in medical preparations containing active ingredients, pharmaceutical formulations, peptide/protein components, etc., can solve the problem of decreased white blood cell count, odorless or stale, increased, mucosal congestion, redness and swelling and other problems, to achieve good application prospects, significant curative effects, and significant therapeutic effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
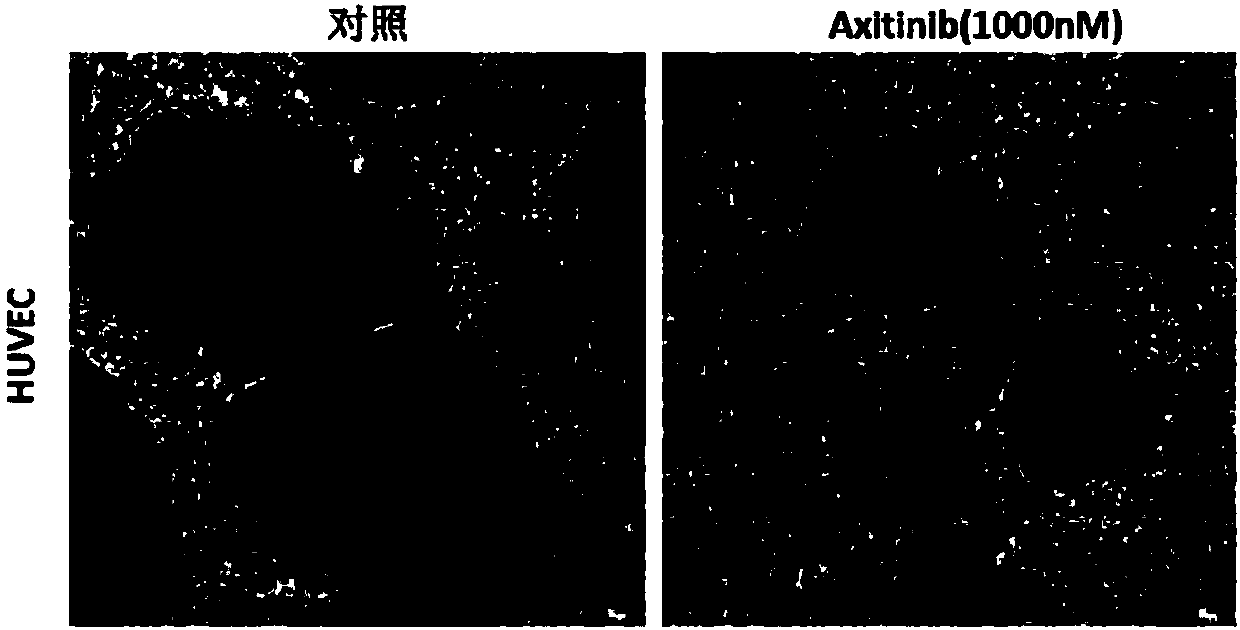
[0030] Example 1 Effect of axitinib on tube formation of vascular endothelial cells
[0031] 1. Experimental materials
[0032] (1) Drug: Axitinib, its chemical formula is: C22H18N4OS, and its CAS number is: 319460-85-0
[0033] (2) Vascular endothelial cells: human umbilical vein endothelial cells (HUVEC)
[0034] (3) Commercially available matrigel.
[0035] 2. Experimental grouping
[0036] (1) Control group: blank control, that is, HUVEC without any drug treatment.
[0037] (2) Experimental group: HUVEC were treated with axitinib.
[0038] 3. Angiogenesis assay to detect the effect of axitinib on HUVEC tube formation
[0039] (1) After thawing Matrigel at -20°C, spread 30 μL on a 96-well plate and place in a 37°C incubator for 30 minutes;
[0040] (2) Digest HUVEC cells in the logarithmic growth phase with trypsin and resuspend in the complete medium, take 200 μL (containing 5×10 4 Cells) were spread evenly on Matrigel matrigel in 96-well plate;
[0041] (3) Add ax...
Embodiment 2
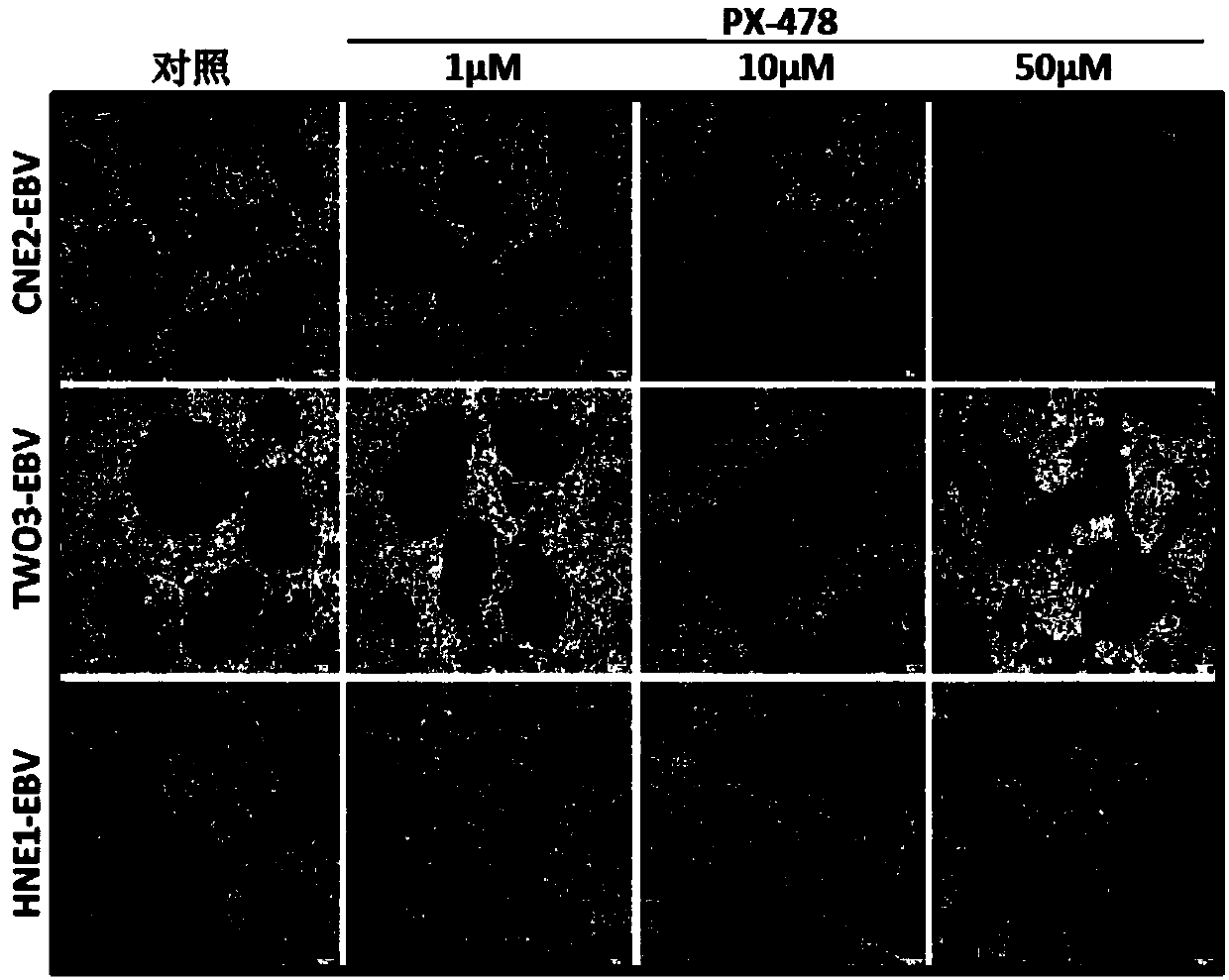
[0045] Example 2 Effect of PX-478 on EBV-positive nasopharyngeal carcinoma cells VM
[0046] 1. Experimental materials
[0047] (1) Drug: PX-478, its chemical formula is: C13H20Cl4N2O3, and its CAS number is: 685898-44-6
[0048] (2) Cancer cells: EBV positive nasopharyngeal carcinoma cells (HNE1-EBV, CNE2-EBV and TWO3-EBV)
[0049] (3) Commercially available matrigel.
[0050] 2. Experimental grouping
[0051] (1) Control group: blank control, that is, the cancer cells were not treated with any drugs.
[0052] (2) Experimental group: Cancer cells were treated with PX-478.
[0053] 3. Angiogenesis assay to detect the effect of PX-478 on EBV-positive nasopharyngeal carcinoma cells VM
[0054] (1) After thawing Matrigel at -20°C, spread 30 μL on a 96-well plate and place in a 37°C incubator for 30 minutes;
[0055] (2) Digest the HNE1-EBV, CNE2-EBV and TWO3-EBV cells in the logarithmic growth phase with trypsin and resuspend in the complete medium, take 200 μL (containing ...
Embodiment 3
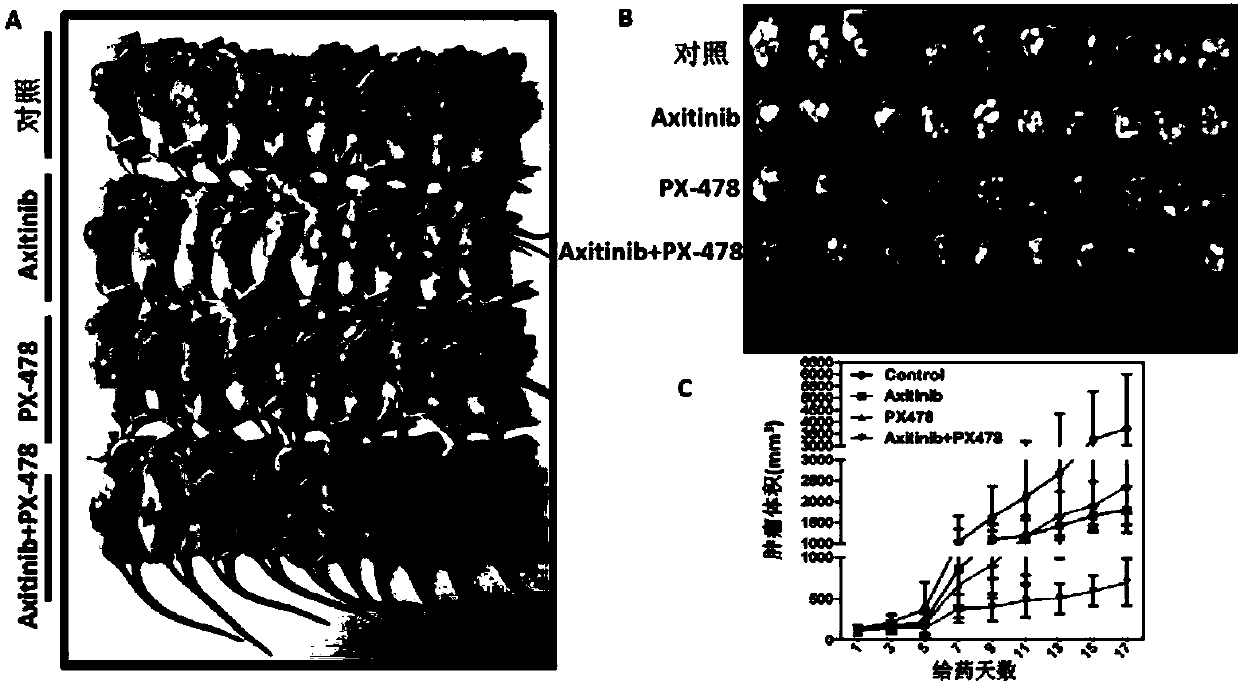
[0060] Example 3 Effect of axitinib combined with PX-478 on EBV-positive nasopharyngeal carcinoma cell xenografts
[0061] 1. Experimental materials
[0062](1) Drugs: Axitinib and PX-478
[0063] (2) Cancer cells: EBV-positive nasopharyngeal carcinoma cells (CNE2-EBV)
[0064] (3) Commercially purchased nude mice.
[0065] 2. Experimental grouping
[0066] (1) Control group: blank control, that is, the cancer cells were not treated with any drugs.
[0067] (2) Axitinib group: Axitinib was used to treat EBV-positive nasopharyngeal carcinoma cells.
[0068] (3) PX-478 group: EBV-positive nasopharyngeal carcinoma cells were treated with PX-478.
[0069] (4) Axitinib + PX-478 group: EBV-positive nasopharyngeal carcinoma cells were treated with axitinib and PX-478 at the same time.
[0070] 3. The effect of axitinib or / and PX-478 on EBV-positive nasopharyngeal carcinoma cell xenografts was detected by subcutaneous tumor formation experiment in nude mice
[0071] (1) with 5×...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com