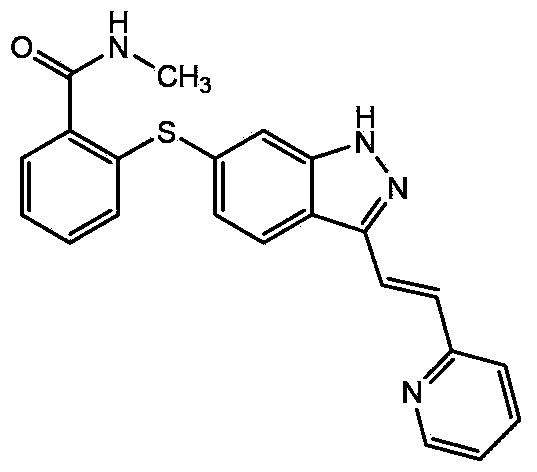
Axitinib crystal form preparation method
A crystal form, axitinib technology, applied in the field of preparation of axitinib crystal form, can solve problems such as difficulties, dangers, and high solvent viscosity, and achieve the effect of low production equipment requirements, high safety factor, and easy operation and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
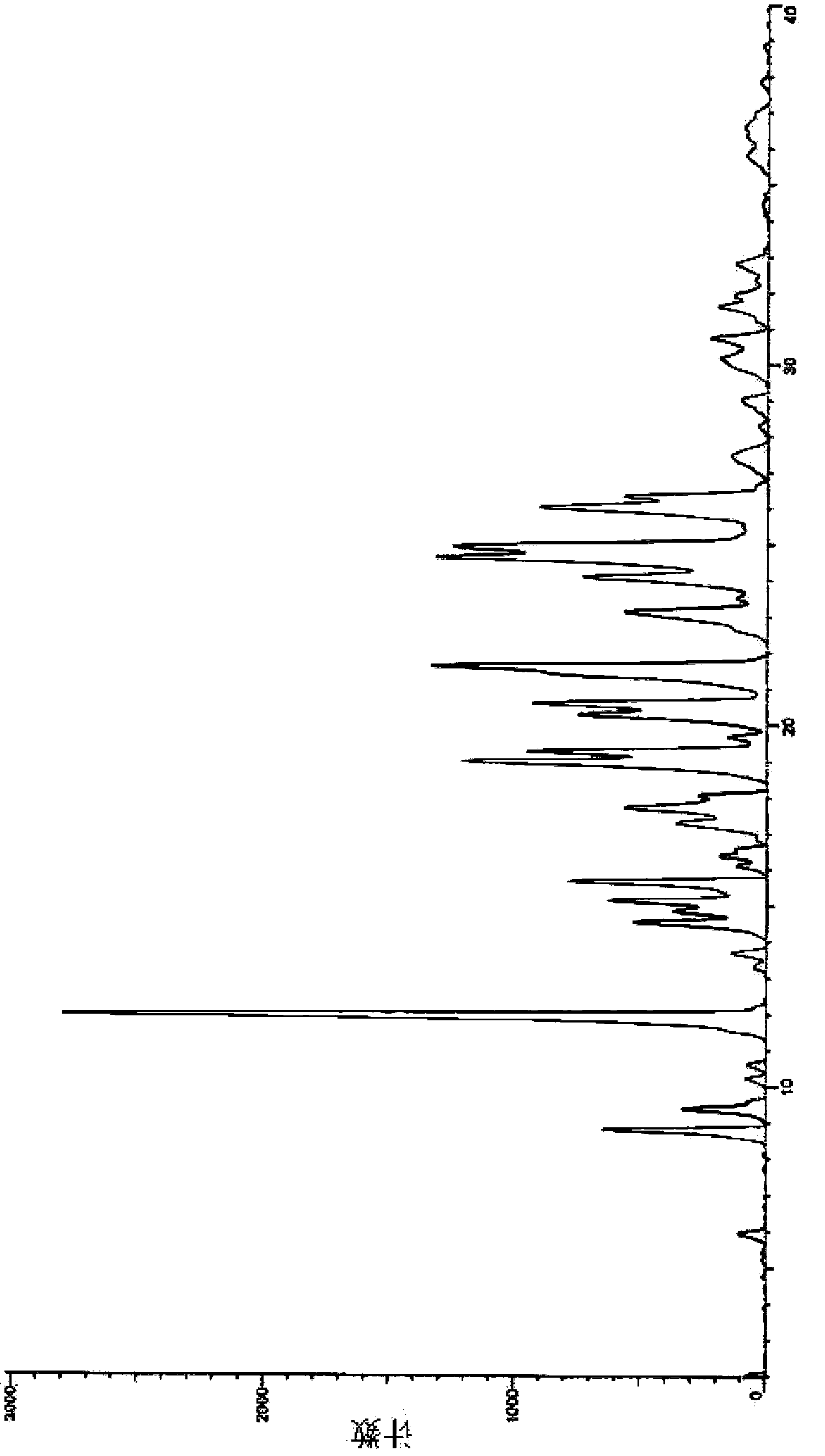
Image
Examples
reference example 1
[0024] Reference Example 1: Refer to the preparation method of crystal form I provided by patent WO2006048751A1 to prepare axitinib crystal form I. The method is: the 6-[2- (Methylcarbamoyl)phenylsulfanyl]-3-E-[2-(pyridin-2-yl)vinyl]indazole (4.6 g) was slurried in 50 ml methanol at 50 °C, After 15 minutes, 50ml of water was added, the slurry was stirred well and allowed to cool to room temperature. The solid was collected by filtration, washed successively with 50 ml of water and 30 ml of ethyl acetate, and dried under high vacuum to obtain crystalline form I.
reference example 2
[0025] Reference Example 2: Refer to the preparation method of crystal form II provided by patent WO2006048751A1 to prepare axitinib crystal form II. The crystal form is hydrate.
reference example 3
[0026] Reference Example 3: Refer to the preparation method of ethyl acetate solvate provided by patent WO2006048751A1 to prepare axitinib ethyl acetate solvate (that is, the crystal form III described in the patent), and neutralize axitinib in ethyl acetate p-Toluenesulfonate derivative, the resulting solid was dried under vacuum at 65°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com