Pharmaceutical composition for treating renal clear cell carcinoma and application thereof
A technology for renal clear cell carcinoma and therapeutic drugs, applied in the field of medicine, can solve problems such as drug resistance that damages anti-cancer efficacy, and no research reports have been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
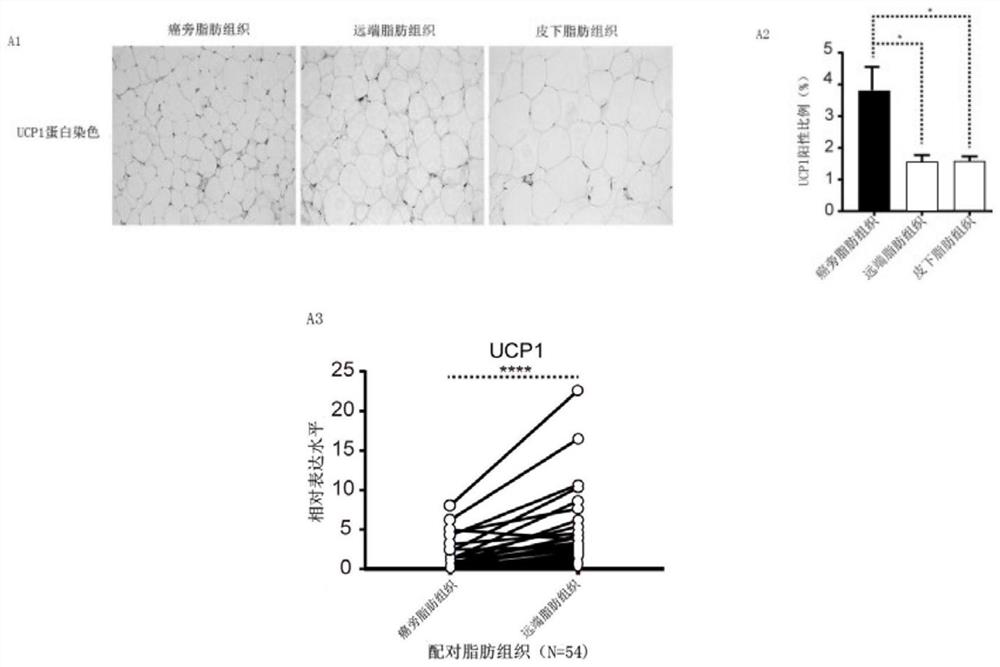
[0030] In this example, it was discovered by analyzing the fat browning marker protein UCP1 in the cancer and paracancerous adipose tissue sections of patients with renal cancer clinically. The analysis results are as follows figure 1 As shown, the degree of browning of the proximal adipose tissue of RCC is stronger than that of the distal and subcutaneous adipose tissue, and there are significant differences. In addition, clinically, there is a significant difference in the expression of UCP1 protein between cancer and adjacent tumors in patients with RCC, and the adjacent adipose tissue is significantly higher than the distal end, which proves that RCC tumors can promote the browning of adjacent white adipose tissue. Previous studies have demonstrated that browned adipose tissue can secrete free fatty acids, which are conducive to tumor growth. Therefore, RCC can alter the tumor microenvironment by affecting the surrounding white adipose tissue.
Embodiment 2
[0032] This example verifies that the four most clinically used anti-tumor drugs for RCC (sunitinib, sorafenib, axitinib, and pazopanib) have the effect of promoting fat browning.
[0033] The specific experimental steps are as follows:
[0034] Step 1: Treat C3H10T1 / 2 cells with 1 μmol of four first-line anti-kidney cancer drugs for six days;
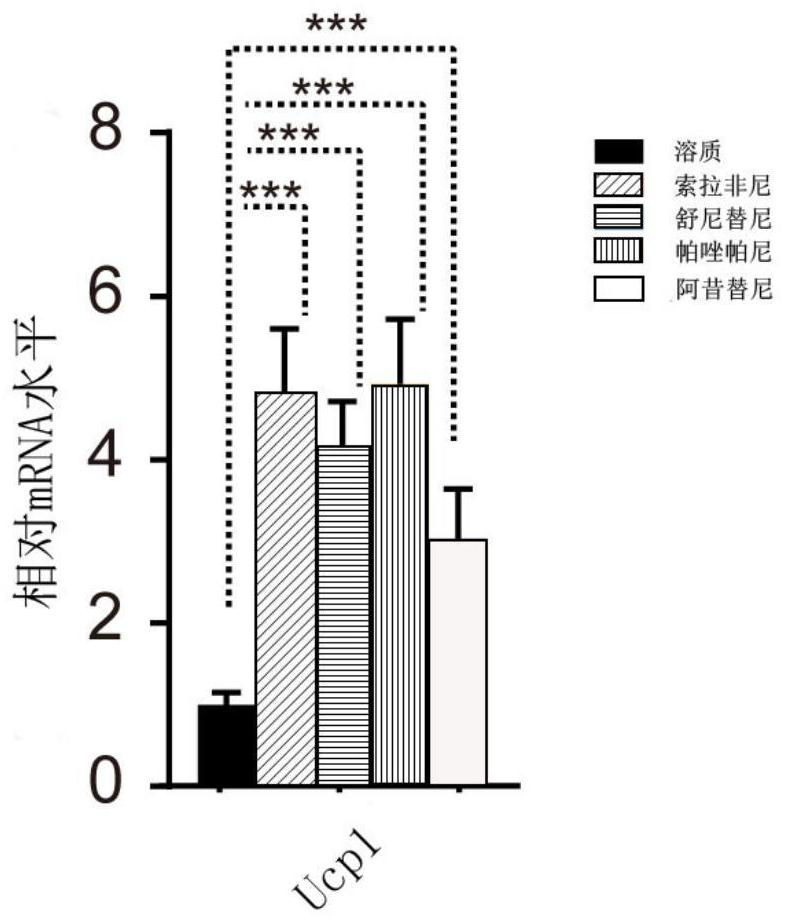
[0035] Step 2: After the cells have completed differentiation, detect the relative expression of UCP1 protein in the experimental group and the control group (such as figure 2 shown).
[0036] Depend on figure 2 It can be seen that the expression level of UCP1 in cells treated with four anticancer drugs was significantly higher than that in the control group, indicating that the clinical treatment of four anticancer drugs can cause browning of adjacent adipose tissue. Previous studies have demonstrated that browned adipose tissue can secrete free fatty acids, which are conducive to tumor growth. Therefore, this example proves tha...
Embodiment 3
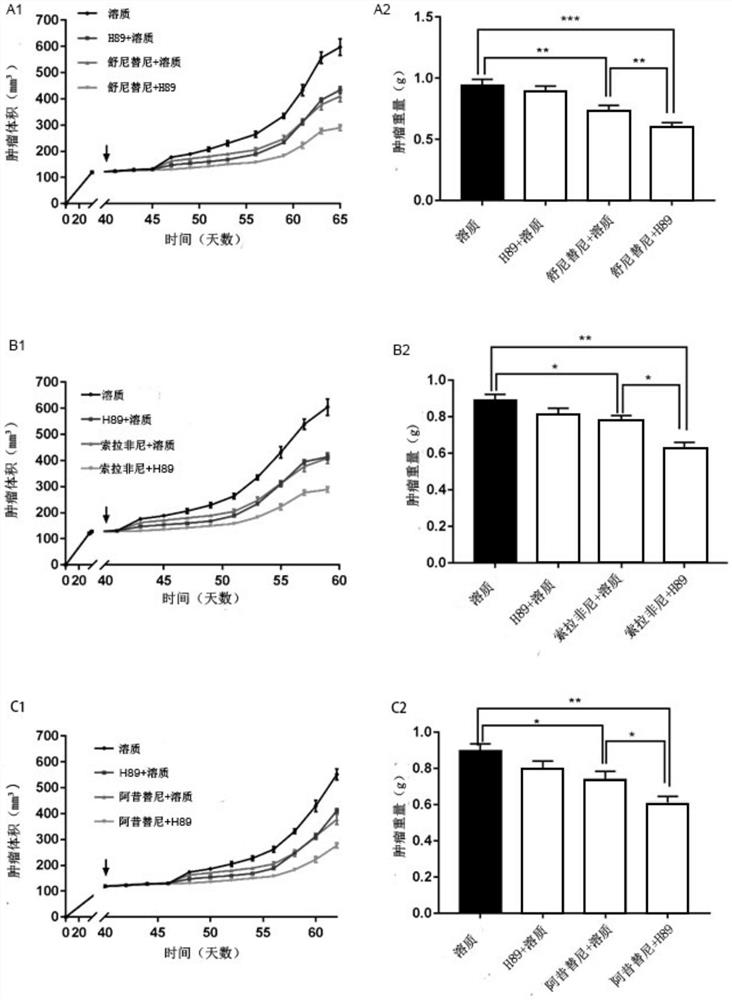
[0038] This example verifies the therapeutic effects of four pharmaceutical compositions including the fat browning inhibitor H89 and first-line kidney cancer treatment drugs, wherein the four pharmaceutical compositions respectively include the following dosage components:
[0039] 1) Fat browning inhibitor H89 is used in combination with sunitinib, the dose of fat browning inhibitor is 1 mg / kg, and the dose of sunitinib is 10 mg / kg;
[0040] 2) Fat browning inhibitor H89 is used in combination with sorafenib, the dose of fat browning inhibitor is 1 mg / kg, and the dose of sorafenib is 10 mg / kg;
[0041] 3) Fat browning inhibitor H89 is used in combination with axitinib, the dose of fat browning inhibitor is 1 mg / kg, and the dose of axitinib is 10 mg / kg;
[0042] 4) Fat browning inhibitor H89 is used in combination with pazopanib, the dose of fat browning inhibitor is 1 mg / kg, and the dose of pazopanib is 10 mg / kg.
[0043] The specific experimental steps are as follows:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com