Application of axitinib in treating nasopharynx cancer
A technology for axitinib and nasopharyngeal carcinoma, which is applied in the application field of axitinib in the treatment of nasopharyngeal carcinoma, and can solve atrophic fibrosis, radioactive dental caries, radioactive jaw osteomyelitis and radioactive cerebrospinal cord disease, decreased white blood cell count, tasteless or changed taste, etc., to achieve a solid clinical application foundation, good application prospects, and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
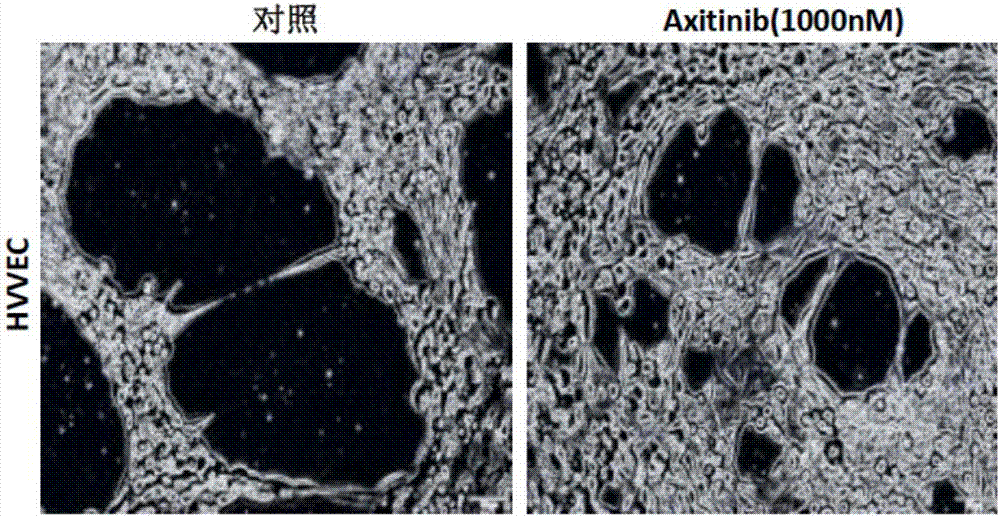
[0024] Example 1 Effect of axitinib on tube formation of vascular endothelial cells
[0025] 1. Experimental materials
[0026] (1) Drug: Axitinib, its chemical formula is: C22H18N4OS, and its CAS number is: 319460-85-0
[0027] (2) Vascular endothelial cells: human umbilical vein endothelial cells (HUVEC)
[0028] (3) Commercially available matrigel.
[0029] 2. Experimental grouping
[0030] (1) Control group: blank control, that is, HUVEC without any drug treatment.
[0031] (2) Experimental group: HUVEC were treated with axitinib.
[0032] 3. Angiogenesis assay to detect the effect of axitinib on HUVEC tube formation
[0033] (1) After thawing Matrigel at -20°C, spread 30 μL on a 96-well plate and place in a 37°C incubator for 30 minutes;
[0034] (2) Digest HUVEC cells in the logarithmic growth phase with trypsin and resuspend in the complete medium, take 200 μL (containing 5×10 4 Cells) were spread evenly on Matrigel matrigel in 96-well plate;
[0035] (3) Add ax...
Embodiment 2
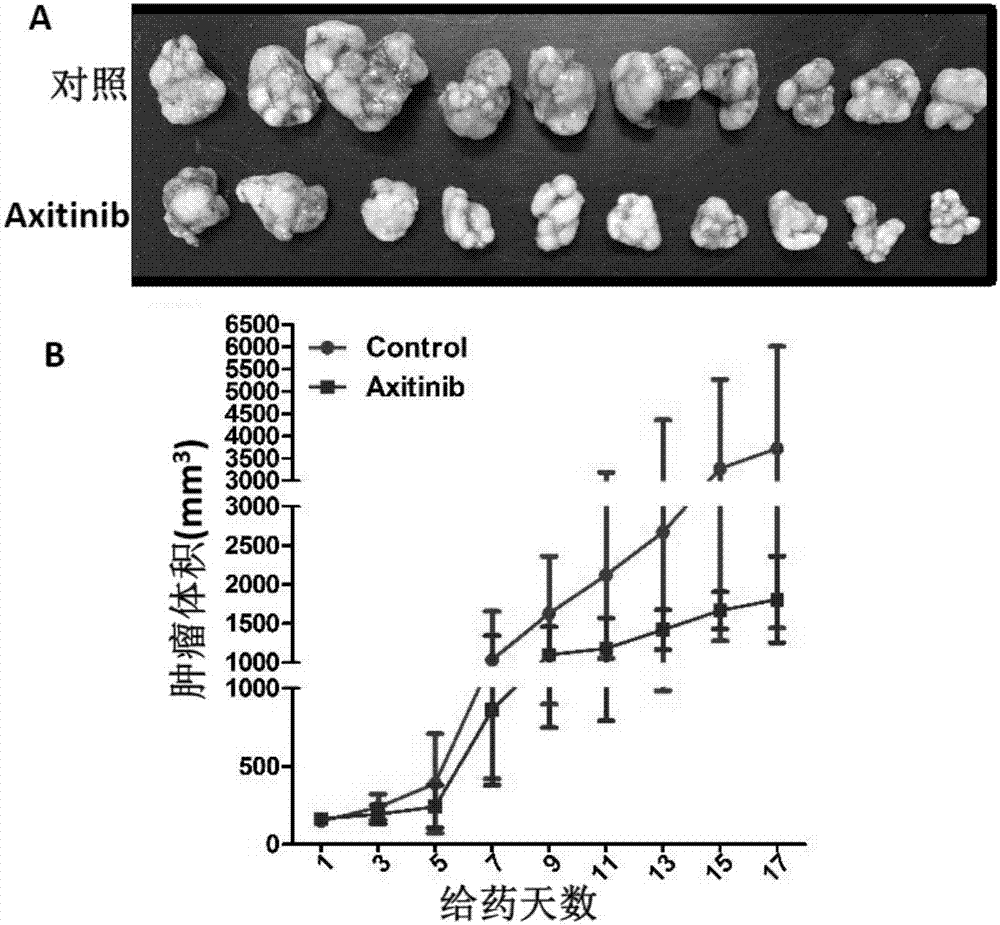
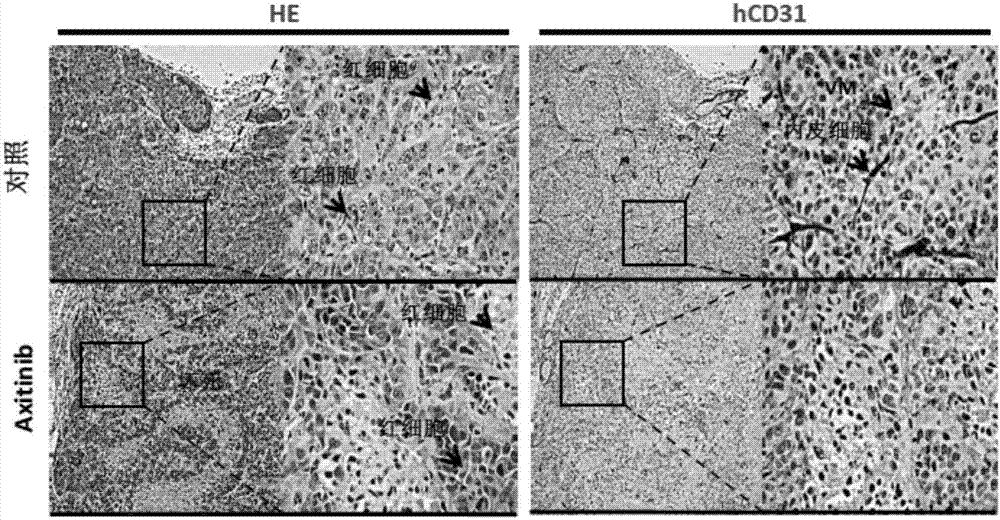
[0039] Example 2 Effect of Axitinib on EBV-positive Nasopharyngeal Carcinoma Transplanted Tumor
[0040] 1. Experimental materials
[0041] (1) Drug: Axitinib
[0042] (2) Cancer cells: EBV-positive nasopharyngeal carcinoma cells (CNE2-EBV)
[0043] (3) Commercially purchased nude mice.
[0044] 2. Experimental grouping
[0045] (1) Control group: blank control, that is, the cancer cells were not treated with any drugs.
[0046] (2) Axitinib group: Axitinib was used to treat EBV-positive nasopharyngeal carcinoma cells.
[0047] 3. Subcutaneous tumor formation experiment in nude mice to detect the effect of axitinib on EBV-positive nasopharyngeal carcinoma cell xenografts
[0048] (1) with 5×10 6 Numerous EBV-positive nasopharyngeal carcinoma cells (CNE2-EBV) were implanted subcutaneously in the axilla of 40 NOD / SCID mice aged 3-4 weeks. They were randomly divided into 2 groups: blank control group and axitinib group.
[0049] (2) When the size of the subcutaneous tumor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com