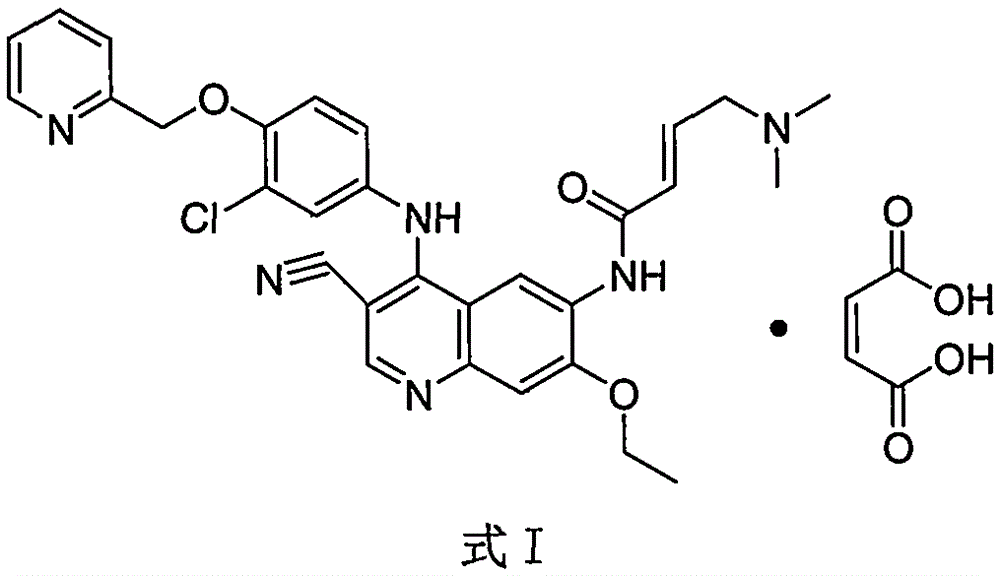
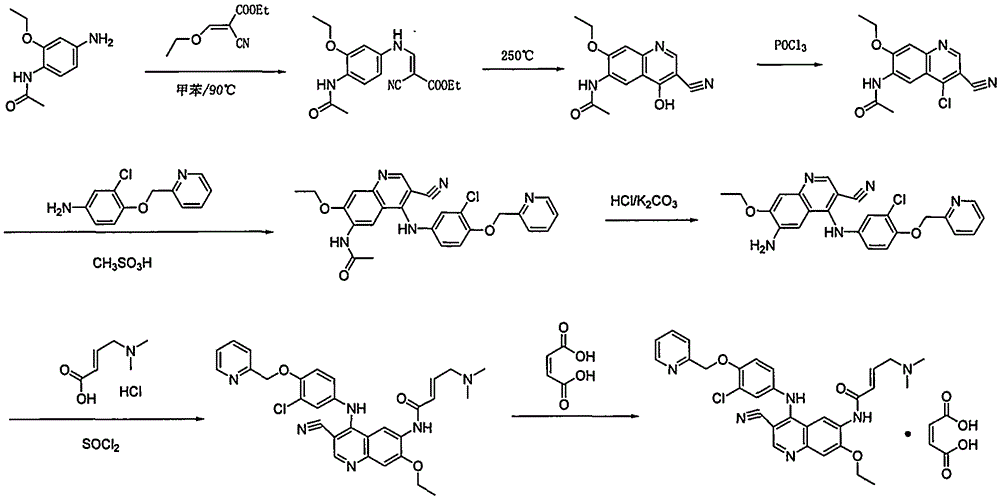
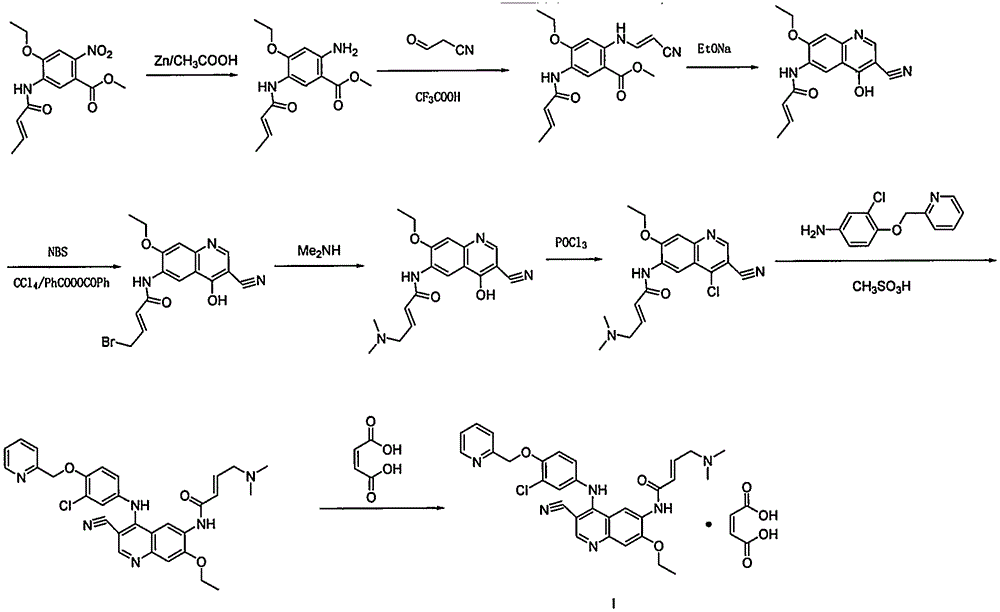
Preparation method of antineoplastic drug maleic acid neratinib
A technology of neratinib and maleic acid, which is applied in the field of chemical preparation of the antineoplastic drug neratinib maleate, can solve the problem of unfavorable personnel and environment for the chlorination agent phosphorus oxychloride, difficult source of raw materials, and total synthesis. Low yield and other problems, to achieve the effect of convenient post-reaction treatment, high product yield, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 4-(4-((pyridin-2-yl)methoxy)-3-chloroaniline)-7-ethoxy-6-nitro-3-cyanoquinoline (VI):
[0034]
[0035] Under nitrogen protection, compound III 4-amino-7-ethoxy-3-cyano-6-nitroquinoline (2.58g, 10mmol) was suspended in absolute ethanol, and compound II (4-bromo-2-chloro Phenoxy)methyl-2-pyridine (2.99g, 10mmol) and methanesulfonic acid (48mg, 0.5mmol) were reacted at 70°C for 2 hours, and the reaction liquid gradually became clear and solid precipitated out. After heating stopped, cool to room temperature, filter, wash with 50% ethanol, and dry to obtain 4.38 g of the title compound with a yield of 92%. ESI-MS: [M+H] + =476.79, 1 HNMR-δ(CDCl 3 ) / 300MHz): 1.6(t, 3H, J=6.8, 13.7), 4.3(q, 2H, J=7.2, 13.8), 5.3(s, 2H), 6.1(d, 1H, J=15.0), 6.3 (d, 1H, J=15.0), 7.3(s, 1H), 7.4(s, 1H), 7.6(d, IH, J=8.2), 7.8(d, 1H, J=7.6), 8.0(s, 1H), 8.5 (s, 1H), 8.6 (d, 1H, J=4.7), 9.2 (s, 1H).
Embodiment 2
[0036] Example 2: Preparation of 4-(4-((pyridin-2-yl)methoxy)-3-chloroaniline)-7-ethoxy-6-nitro-3-cyanoquinoline (VI):
[0037] Under nitrogen protection, compound III 4-amino-7-ethoxy-3-cyano-6-nitroquinoline (2.58g, 10mmol) was suspended in anhydrous methanol, and compound II (2,4-dichlorobenzene Oxy)methyl-2-pyridine (2.54g, 10mmol) and trifluoroacetic acid (57mg, 0.5mmol) were reacted at 20°C for 4 hours, and the reaction liquid gradually became clear and solid precipitated out. After heating stopped, cool to room temperature, filter, wash with 50% methanol, and dry to obtain 4.09 g of the title compound with a yield of 86%. ESI-MS: [M+H] + =476.79, 1 HNMR-δ(CDCl 3 ) / 300MHz): 1.6(t, 3H, J=6.8, 13.7), 4.3(q, 2H, J=7.2, 13.8), 5.3(s, 2H), 6.1(d, 1H, J=15.0), 6.3 (d, 1H, J=15.0), 7.3(s, 1H), 7.4(s, 1H), 7.6(d, 1H, J=8.2), 7.8(d, 1H, J=7.6), 8.0(s, 1H), 8.5 (s, 1H), 8.6 (d, 1H, J=4.7), 9.2 (s, 1H).
Embodiment 3
[0038] Example 3: Preparation of 4-(4-((pyridin-2-yl)methoxy)-3-chloroaniline)-7-ethoxy-6-nitro-3-cyanoquinoline (VI):
[0039] Under nitrogen protection, compound III 4-amino-7-ethoxy-3-cyano-6-nitroquinoline (2-58g, 10mmol) was suspended in anhydrous DMF, and compound II (4-trifluoromethanesulfonate Acyloxy-2-chlorophenoxy)methyl-2-pyridine (3.68g, 10mmol) and sulfuric acid (50mg, 0.5mmol) were reacted at 150°C for 1 hour, and the reaction liquid gradually became clear and solid precipitated out. After heating stopped, cool to room temperature, filter, wash with 50% ethanol, and dry to obtain 4.28 g of the title compound with a yield of 90%. ESI-MS: [M+H] + =476.79, 1 HNMR-δ(CDCl 3 ) / 300MHz): 1.6(t, 3H, J=6.8, 13.7), 4.3(q, 2H, J=7.2, 13.8), 5.3(s, 2H), 6.1(d, 1H, J=15.0), 6.3 (d, 1H, J=15.0), 7.3(s, 1H), 7.4(s, 1H), 7.6(d, 1H, J=8.2), 7.8(d, 1H, J=7.6), 8.0(s, 1H), 8.5 (s, 1H), 8.6 (d, 1H, J=4.7), 9.2 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com