Preparation method of neratinib
A technology of neratinib and tinib hydrochloride, which is applied in the field of preparation of neratinib, can solve the problems of large amount of dimethylaminocroton hydrochloride, unfavorable industrial production, lengthy reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
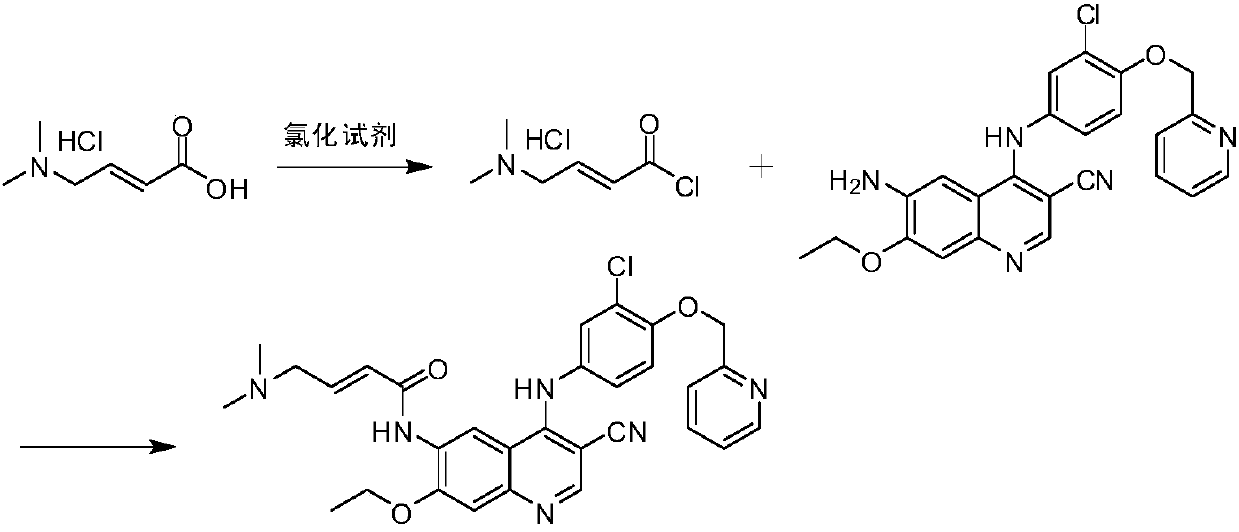
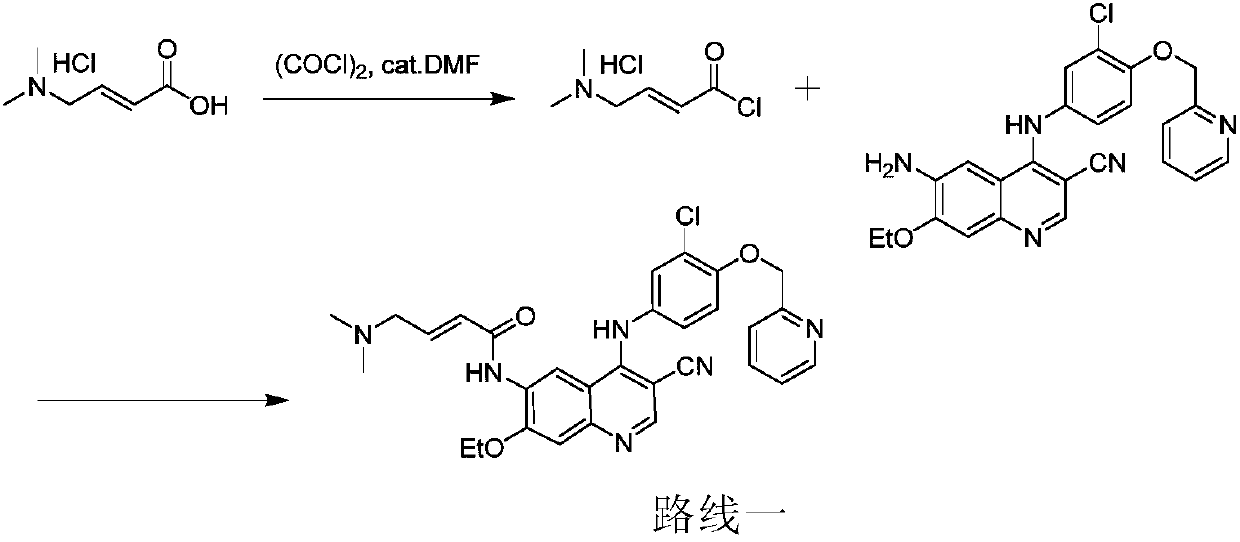
[0057] The preparation method of Neratinib of the present invention may comprise the following steps:
[0058]
[0059] (1) Dissolve trans-4-dimethylaminocrotonyl chloride hydrochloride in organic solvent 1, and then react with a chlorination reagent to obtain trans-4-dimethylaminocrotonyl chloride salt solution;
[0060] (2) Dissolve 6-amino-4-[[3-chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinoline in In the organic solvent 2, then dropwise added to the solution obtained in step (1) to react, thereby obtaining neratinib hydrochloride;
[0061] (3) Dissolving neratinib hydrochloride obtained in step (2) in water and an organic solvent 3, adjusting the pH value to 7-10, and reacting to obtain neratinib.
[0062] In the step (1), the chlorination reagent is selected from the group consisting of thionyl chloride, oxalyl chloride, phosphorus oxychloride or combinations thereof, preferably thionyl chloride.
[0063] In the step (1), the molar ratio of the ...
Embodiment 1
[0081] The preparation of neratinib described in embodiment 1 present invention
[0082] (1) Add 10g of trans-4-dimethylaminocroton hydrochloride and 60ml of dimethyl sulfoxide to a 250ml three-neck flask, stir at room temperature, add 10.8g of thionyl chloride dropwise into a constant pressure dropping funnel, for 1h After the dropwise addition, the reaction temperature was controlled at -25 to -20°C. During the reaction, the solution gradually changed from white to light brown, monitored by HPLC, and the reaction was stopped after 2 hours, and the obtained reaction solution was used for later use;
[0083] (2) Add 18 g of 6-amino-4-[[3-chloro-4-[(pyridine-2- Base) methoxy] phenyl] amino] -3-cyano-7-ethoxyquinoline (also known as aminoquinoline) 70ml N-methylpyrrolidone solution, 1h dropwise, the reaction temperature is controlled at -25~-20 ℃, insulation reaction 4h, HPLC detects (at this moment, the content of aminoquinoline in the reaction solution is 0.08%, and its reten...
Embodiment 2
[0085] Embodiment 2 The preparation of Neratinib according to the present invention
[0086] (1) Add 10g of trans-4-dimethylamino croton hydrochloride and 80ml of N,N-dimethylacetamide to a 250ml three-necked flask, stir at room temperature, add 10.8g of chlorine The addition of sulfoxide was completed in 1 hour, and the reaction temperature was controlled at -15 to -10°C. During the reaction, the solution gradually changed from white to light brown, monitored by HPLC, and the reaction was stopped after 2 hours, and the obtained reaction solution was used for later use;
[0087] (2) Add 18 g of 6-amino-4-[[3-chloro-4-[(pyridine-2- base) methoxy] phenyl] amino] -3-cyano-7-ethoxyquinoline in 70ml of N-methylpyrrolidone solution, after 1h dropwise addition, the reaction temperature was controlled at -15~-10°C and kept warm After reacting for 2 hours, stop the reaction, add 10ml of methanol dropwise, filter with suction, rinse twice with 10ml of dichloromethane, and obtain nerati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com