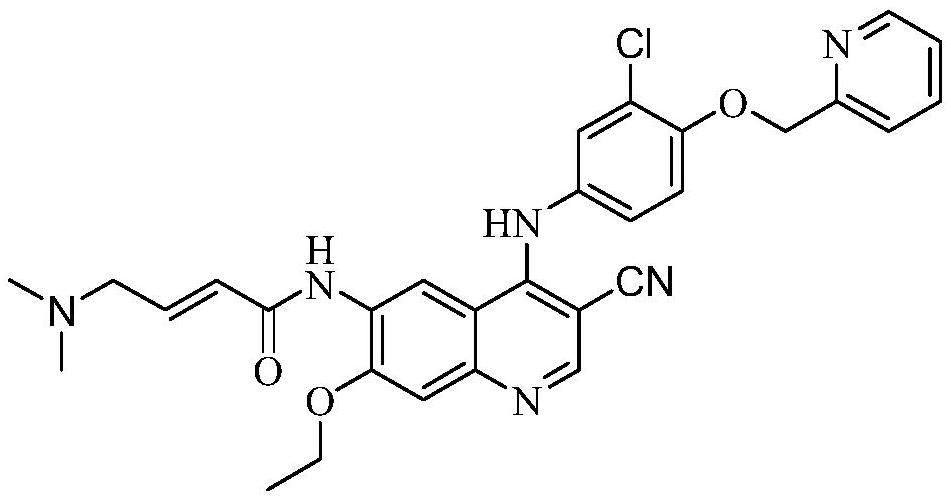
A kind of preparation method of neratinib or its pharmaceutically acceptable salt pharmaceutical composition
A technology of neratinib and solvate, which is applied in the field of preparation of neratinib or its pharmaceutically acceptable salt pharmaceutical composition, can solve the problem of inability to guarantee the stability and good dissolution properties of the final preparation, instability and easy degradation Chemical properties and other issues to achieve good dissolution properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Neratinib maleate, mannitol, microcrystalline cellulose, crospovidone, and silicon dioxide will be granulated in a fluidized bed according to the ratio in Table 1, with 5% polyvinylpyrrolidone as the binding agent. After the granulation is completed, stop spraying the binder and dry the granules. Add sodium stearyl fumarate according to the ratio in Table 1. Mixing is performed using a rotary blender. The obtained blended granules are filled into capsules to prepare capsules.
[0047] Table 1: Prescription of Neratinib Maleate Capsules
[0048] Element Unit dose (mg) Proportion(%) Neratinib maleate (anhydrous) 290 48.33 Mannitol 202 33.67 microcrystalline cellulose 36 6.00 Crospovidone 18 3.00 colloidal silica 12 2.00 Polyvinylpyrrolidone 30 5.00 Sodium stearyl fumarate 12 2.00 total 600 100
[0049] According to the above prescription, the DPL-II fluidized bed (manufactured by Chongqing Jinggo...
Embodiment 2
[0061] Dissolution test results
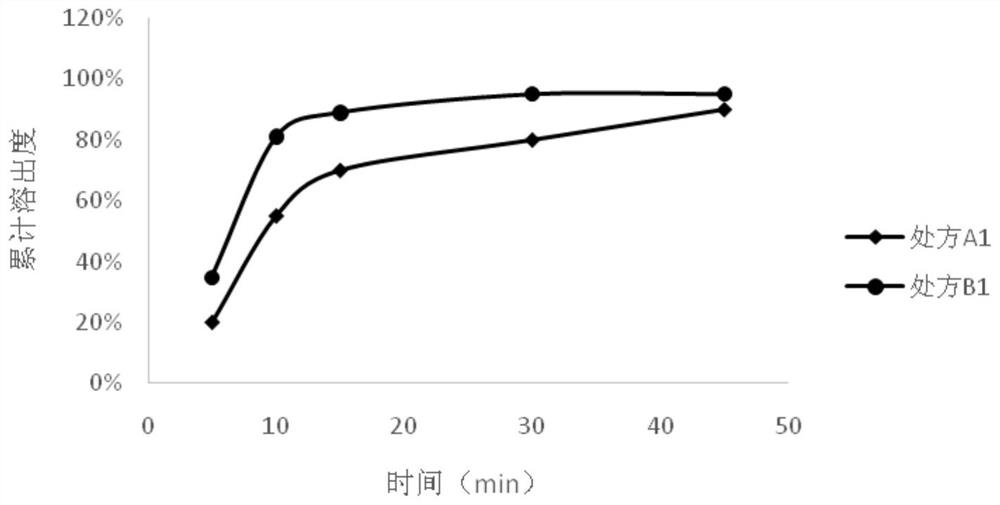
[0062] Neratinib maleate capsules of prescription B1 and prescription A1 of Example 1 were investigated for dissolution rate. In Chinese Pharmacopoeia Dissolution Determination Method 2 Slurry Method, 900mL of hydrochloric acid solution with pH 1.0 was used as the dissolution medium to investigate the dissolution of capsules. The temperature of the dissolution medium was 37±0.5°C, and the paddle speed was 50rpm. Samples were collected at 5, 10, 15, 30, and 45 min, and measured at 266 nm by an ultraviolet spectrometer.
[0063] The dissolution measurement result and the dissolution curve of the neratinib maleate capsule prepared by the method of the present invention are shown in figure 1 middle. The ordinate shows the dissolution rate of neratinib, and the abscissa shows the time (min). The results showed that the neratinib capsules (B1) prepared by this method dissolved rapidly, and more than 80% could be dissolved in 15 minutes, which was...
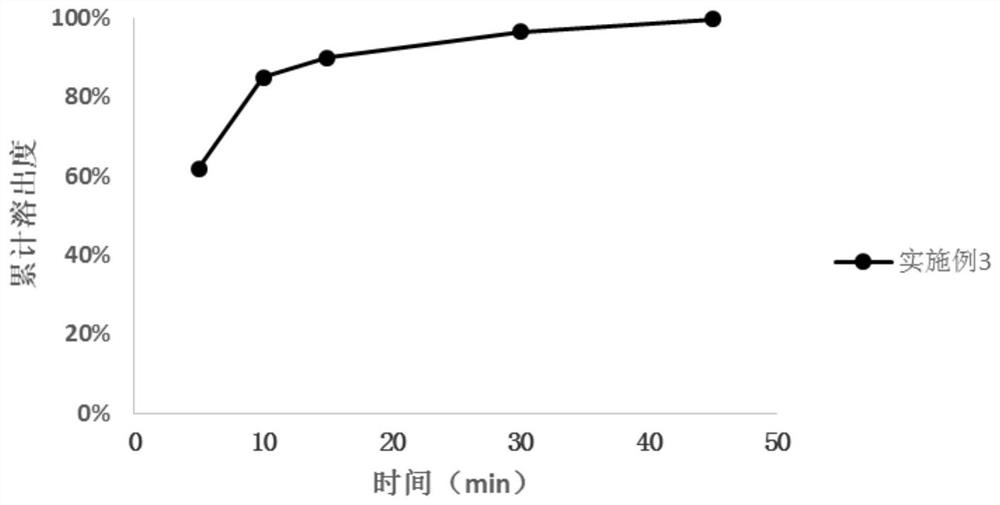
Embodiment 3
[0065] Neratinib, mannitol, microcrystalline cellulose, crospovidone, and silicon dioxide were granulated in a fluidized bed according to the ratio in Table 1, and sprayed into The binder is granulated, and after the granulation is completed, the spraying of the binder is stopped, and the granules are dried. Add sodium stearyl fumarate according to the ratio in Table 1. Mixing is performed using a rotary blender. The obtained blended granules are filled into capsules to prepare capsules.
[0066] Table 5: Prescription of Neratinib Maleate Tablets
[0067] Element Unit dose (mg) Proportion(%) Neratinib maleate (anhydrous) 290 36.25 Mannitol 335 41.88 microcrystalline cellulose 60 7.50 Crospovidone 25 3.13 colloidal silica 16 2.00 Polyvinylpyrrolidone 42 5.25 Crospovidone 16 2.00 Sodium stearyl fumarate 16 2.00 total 800 100
[0068] According to the above prescription, the DPL-II fluidized bed (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com