Preparation method of Neratinib intermediate
A technology of neratinib and intermediates, applied in the field of chemical synthesis, can solve the problems of difficulty in industrialized production, low utilization rate of raw materials, high production cost, etc., and achieve the effect of improving product utilization rate, reducing reaction difficulty, and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
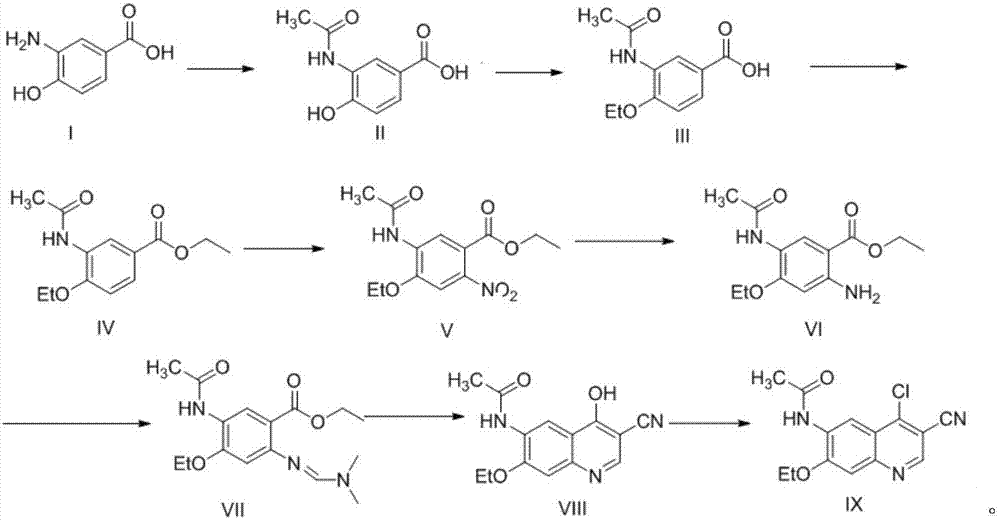
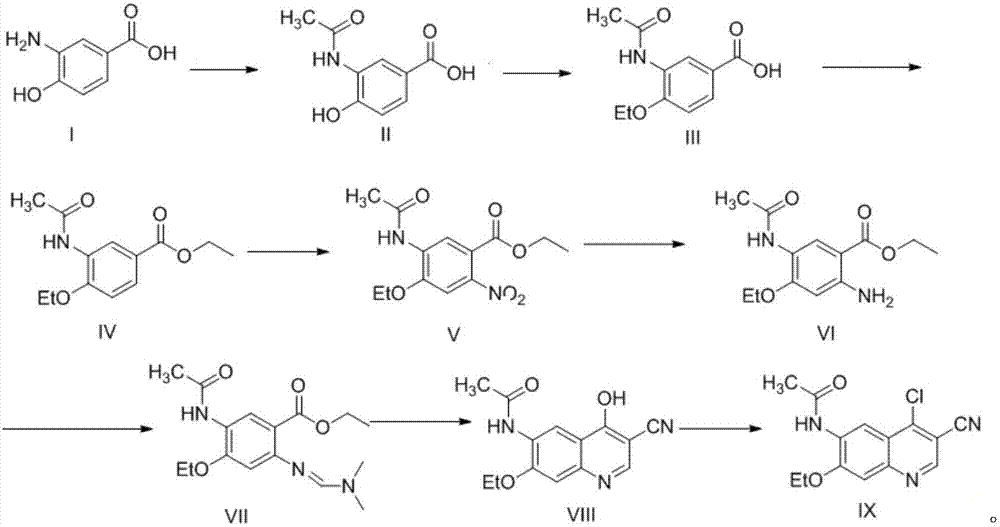
[0036] The specific process of compound II synthesis is as follows: add compound I (1mol) to the reaction flask, dissolve in acetic acid (1L), stir to dissolve, heat up to 60°C, add acetic anhydride (1.5mol) dropwise to the solution, and the dropwise addition is completed , Control the temperature at 60°C for 2h. HPLC detection showed that the raw materials were completely reacted. The reaction solution was lowered to room temperature, poured into water, stirred for 1 h, a solid precipitated out, filtered with suction, and dried in vacuum at 40°C to obtain Compound II (0.96mol) with a yield of 96%.
[0037] The specific process of compound III synthesis is as follows: add compound II (1mol) to the reaction flask, dissolve it in DMF (1L), add potassium carbonate (1.8mol), stir and mix, and heat up to 60-62°C. Bromoethane (1.3 mol) was added dropwise to the system. After the dropwise addition was completed, the temperature was controlled at 60° C. for 2 h. TLC spots the plate,...
Embodiment 2
[0046] Add compound I (1mol) to the reaction flask, dissolve in DMF (1L), stir to dissolve, raise the temperature to 60°C, add acetic anhydride (1mol) dropwise to the solution, and control the temperature at 60°C for 2h. HPLC detection showed that the raw materials were completely reacted. The reaction solution was lowered to room temperature, poured into water, stirred for 1 h, a solid was precipitated, filtered with suction, and dried in vacuum at 40°C to obtain compound II (0.89 mol) with a yield of 89%.
[0047] The synthesis process of compounds III~IX is the same as that in Example 1.
Embodiment 3
[0049] Add compound I (1mol) to the reaction flask, dissolve in acetic acid (500ml) and dichloromethane (500ml), stir to dissolve, heat up to reflux, add acetic anhydride (2mol) dropwise to the solution, after the addition is complete, reflux reaction 2h. HPLC detection showed that the raw materials were completely reacted. The reaction solution was lowered to room temperature, poured into water, stirred for 1 h, a solid precipitated, filtered with suction, and dried in vacuum at 40°C to obtain compound II (0.95 mol) with a yield of 95%.
[0050] The synthesis process of compounds III~IX is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com