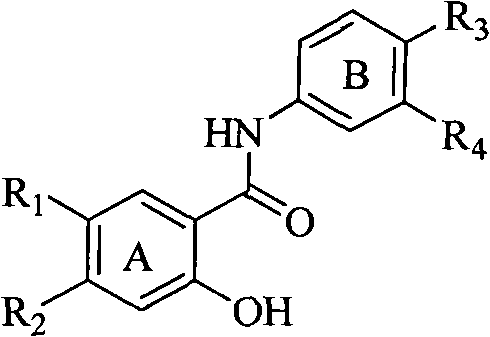
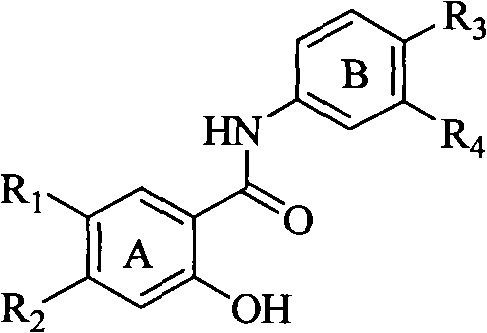
Salicyloyl anilines compound as well as preparation method and application thereof
A technology of salicylanilide and compound, which is applied in the field of pharmaceutical compounds, can solve the problems of being different, cannot increase the curative effect, etc., and achieves the effects of high inhibitory activity and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1, prepare the salicylanilide compound of the following general formula, wherein R1=R2=methoxyethoxyl group, according to the following preparation method:
[0034]
[0035] Synthesis of Intermediate 2:
[0036] Compound 1 (1.0g, 5.5mmol), methoxybromoethane (1.5mL, 16.5mmol) and K 2 CO 3 (2.3g, 16.5mmol) was dissolved in DMF (30mL), heated to 90-100°C and stirred for 9h. The reaction progress was detected by TLC. After the reaction was completed, the DMF was evaporated to dryness. The residue was extracted with diethyl ether (100 mL), and the combined extracts were washed successively with distilled water (30 mL) and saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness to obtain a yellow liquid, which was poured into petroleum ether (20 mL) , placed in a refrigerator to cool, a white solid was precipitated, suction filtered, and the filter cake was dried to obtain 1.4 g of white solid 56, with a...
Embodiment 2
[0055] Example 2, prepare salicylanilide compounds with the following general formula, wherein when R1 is substituted by 5-methanesulfonylethylaminomethylfuran, R2=hydrogen, R4 is hydrogen, chlorine, methyl, methoxy, R3 Take macromolecule substituents or small molecule substituents as an example:
[0056]
[0057] Synthesis of intermediates 17a-h:
[0058] Dissolve 5-iodosalicylic acid 11 (527.0 mg, 2.0 mmol) in tetrahydrofuran (8 mL), then add 7 [or 2.0 mmol of 8, or 9, or 12, or 13, or 14, or 15, or 16 ], and 5 drops of DMF, EDC·HCl (460.0mg, 2.4mmol) and HOAt (327.0mg, 2.4mmol) were added under ice-bath stirring, and the temperature was stirred for 15min and then returned to room temperature and stirred for 24h. The solvent was evaporated under reduced pressure, diluted with dichloromethane (200mL), washed with distilled water (3×100mL) and saturated brine (3×100mL) successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was evaporated to dryness. ...
Embodiment 3
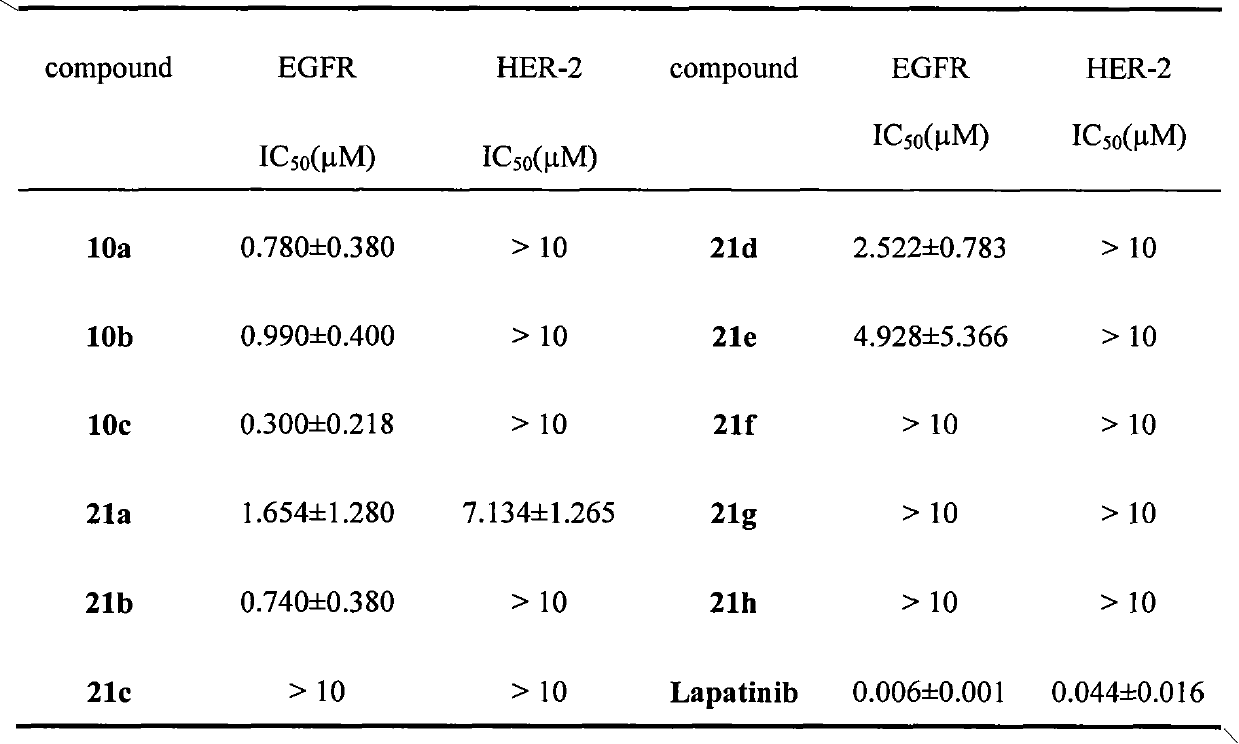
[0087] Embodiment 3, biological activity assay
[0088] With Lapatinib as the positive control drug, the inhibitory activity of the above-mentioned salicylanilide compounds to EGFR and HER-2 was tested, and its half inhibitory concentration (IC 50 ) as shown in the table below:
[0089]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com