Method for analyzing lapatinib ditosylate isomer impurities
A technology of di-p-toluenesulfonic acid and isomers, which is applied in the field of pharmaceutical analysis, can solve the problems that the analysis and detection methods need to be improved, and achieve the effects of short time, high separation efficiency and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: Determination of detection wavelength
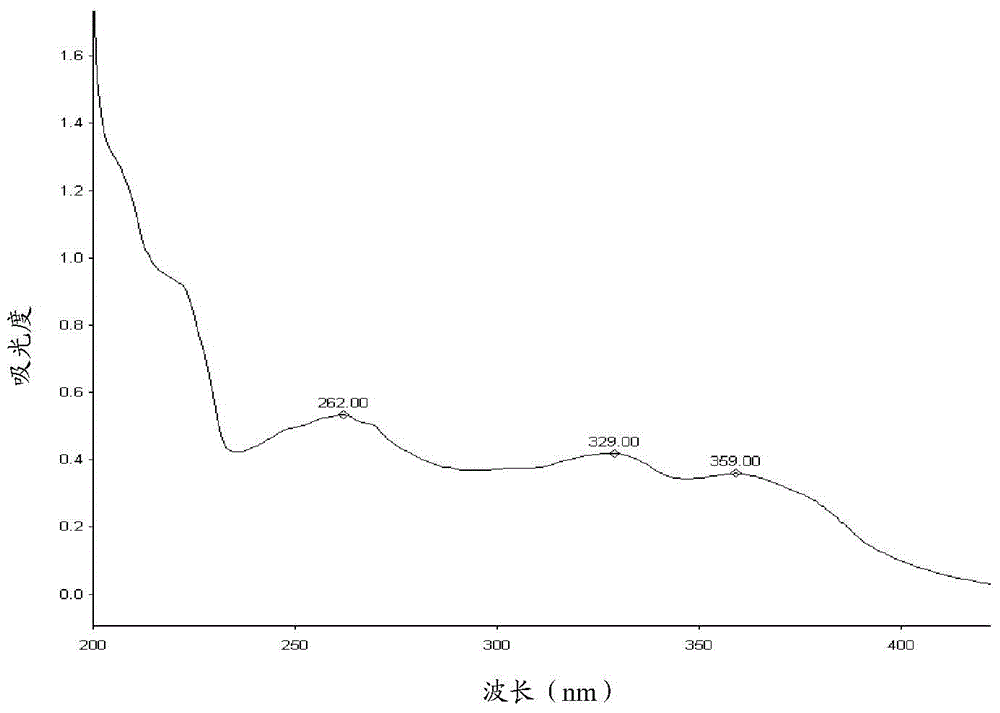
[0063] Take and add acetonitrile-water (volume ratio 4:1) mixed solvent to dissolve and dilute to make a solution containing about 20 μg of raw material per 1 ml, and perform a full scan at 190nm to 400nm on a UV-visible spectrophotometer, di-p-toluenesulfonate The UV scanning results of lapatinib acid are shown in figure 1 . Depend on figure 1 It can be seen that the maximum absorption wavelength of lapatinib di-p-toluenesulfonate is 261nm, so 261±2nm is selected as the detection wavelength.
Embodiment 2
[0065] Using PhenmonexlunaC 18 3μm, 100×4.6mm chromatographic column, use 50mmol / L ammonium acetate buffer solution (take 3.85g ammonium acetate, dissolve in 990ml water, adjust pH to 4.5 with glacial acetic acid, dilute to 1000ml with water) as mobile phase A, use acetonitrile as mobile phase Mobile phase B, the detection wavelength is 261nm, the column temperature is 40°C, and 5 μl of sample is injected, and the linear gradient elution gradient is performed according to the following table:
[0066] time (minutes)
Mobile phase A(%)
Mobile phase B(%)
0
65
35
13
65
35
33
42
58
43
10
90
48
10
90
50
65
35
60
65
35
[0067] Experimental steps:
[0068] 1. Take an appropriate amount of lapatinib di-p-toluenesulfonate, dissolve it with acetonitrile-water (volume ratio 4:1) and quantitatively dilute it with acetonitrile-water (volume ratio 4:1) to make 1m...
Embodiment 3
[0072] (1) Take lapatinib di-p-toluenesulfonate bulk drug, dissolve it ultrasonically in an acetonitrile-water mixed solvent with a volume ratio of 4:1, and prepare lapatinib di-p-toluenesulfonate with a concentration of 0.1mg / ml The test solution;
[0073] Chromatographic conditions:
[0074] The instrument adopts a high-performance liquid chromatograph equipped with an ultraviolet detector; a pentafluorophenyl column PhenmonexKinetex2.6μmPFP100×4.6mm chromatographic column is used, and the ammonium acetate buffer solution with a concentration of 50mmol / L adjusted to pH 4.5 with glacial acetic acid is used as the flow Phase A, using methanol as the mobile phase B, the volume ratio of the ammonium acetate buffer and methanol is 30:70, the elution method is isocratic elution, the detection wavelength is 261nm, the flow rate is 1.0ml / min, the column The temperature is 40 degrees centigrade, and isocratic elution is carried out according to the conditions shown above;
[0075] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com