Process for preparing lapatinib synthetic intermediate
A technology of lapatinib and preparation process, which is applied in the field of preparation of lapatinib synthetic intermediates, can solve the problems of large environmental pollution and difficult post-processing, and achieve the effects of reducing pollution, simple operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
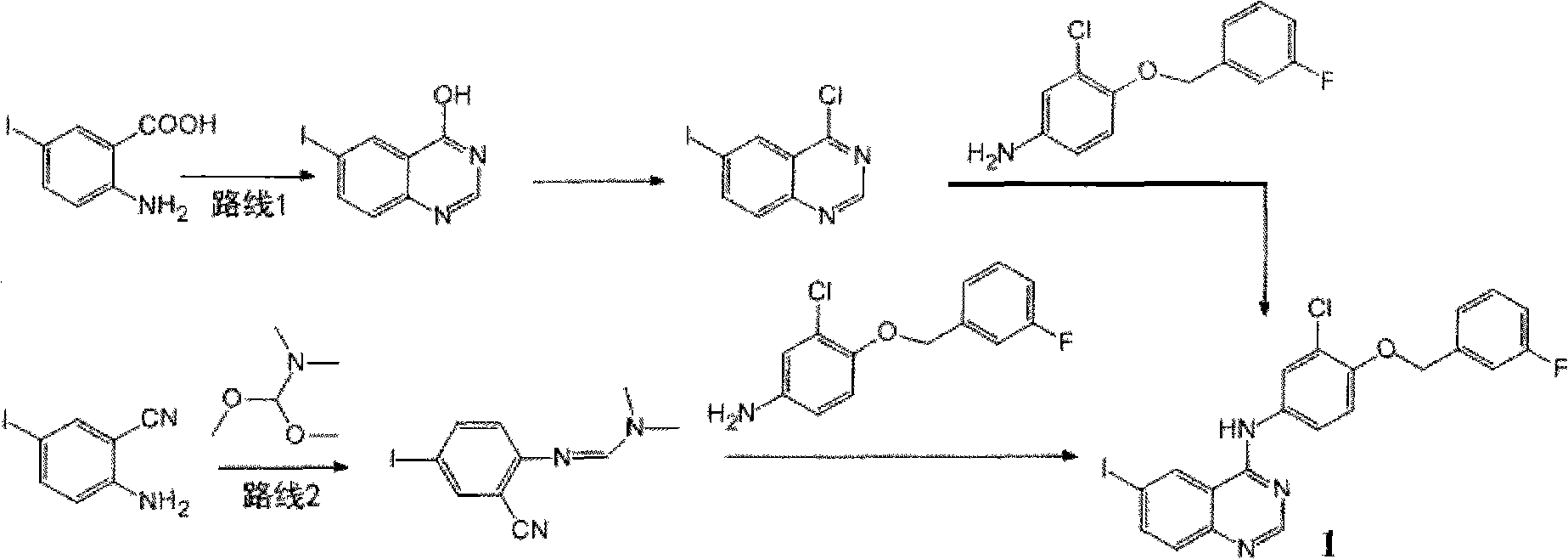
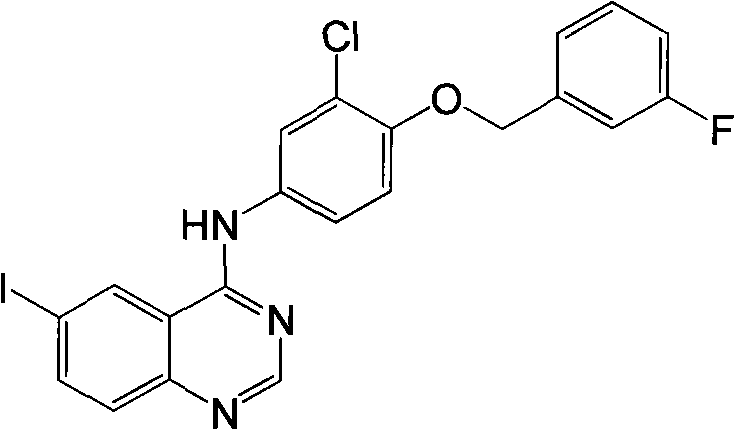
[0014] The preparation of embodiment 1N-(3-chloro-4-(3-fluorobenzyloxy) phenyl)-6-iodoquinazolin-4-amine (1)
[0015] Add 5-iodo-2-aminobenzonitrile (18.4g, 75mmol), DMF-DMA (N,N-dimethylformamide dimethyl acetal, 40ml) into a 100ml eggplant-shaped bottle, heat to reflux at 90-100°C After 1h, excess DMF-DMA was distilled off under reduced pressure, glacial acetic acid (100ml), 3-chloro-4-(3-fluorobenzyloxy)aniline (15g, 59.5mmol) were added and heated to reflux for 1h, cooled to room temperature, poured Pour into ice water (500ml), filter with suction, wash the filter cake with ice water (about 500ml), wash with methanol (1L), and vacuum dry to obtain 124.8g of light yellow solid, yield 82.4%.
[0016] 1 H NMR (DMSO-d6, 300Hz): 5.26(s, 2H); 7.18(m, 1H); 7.34-7.26(m, 3H); 7.47(m, 1H); 7.56(d, J=8.7Hz, 1H ); 7.75(d, J=8.8Hz, 1H); 8.03(s, 1H); 8.11(d, J=8.6Hz, 1H); 8.61(s, 1H); 8.95(s, 1H); 9.84(s ,1H); 13 C NMR (DMSO-d6, 300MHz): 162.2 (d, J=970Hz), 156.3, 154.7, 149.7, 148....
Embodiment 2
[0017] The preparation of embodiment 2N-(3-chloro-4-(3-fluorobenzyloxy) phenyl)-6-iodoquinazolin-4-amine (1)
[0018] Mix 5-iodo-2-aminobenzonitrile and DMF-DMA, the molar ratio of 5-iodo-2-aminobenzonitrile and DMF-DMA is 1:1-5, heat and reflux at 90-100°C for 1h, 0.1MPa / 70-80°C under reduced pressure distillation for 30 minutes to remove excess DMF-DMA, add glacial acetic acid, 3-chloro-4-(3-fluorobenzyloxy)aniline, based on the mass of 5-iodo-2-aminobenzonitrile, The feeding amount of the glacial acetic acid is 1-40ml / g, and the molar ratio of 3-chloro-4-(3-fluorobenzyloxy)aniline and 5-iodo-2-aminobenzonitrile is 1: 1-3, Heat to reflux at 90-100°C for 1h, cool to room temperature, pour into ice water, filter with suction, wash the filter cake with ice water, then methanol, and dry in vacuo to obtain light yellow solid N-(3-chloro-4-(3 -fluorobenzyloxy)phenyl)-6-iodoquinazolin-4-amine.
[0019] The molar ratio of 5-iodo-2-aminobenzonitrile and DMF-DMA is preferably 1:2-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com