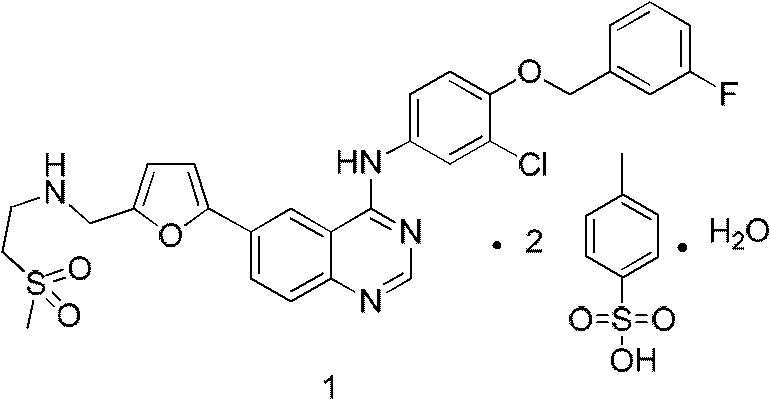
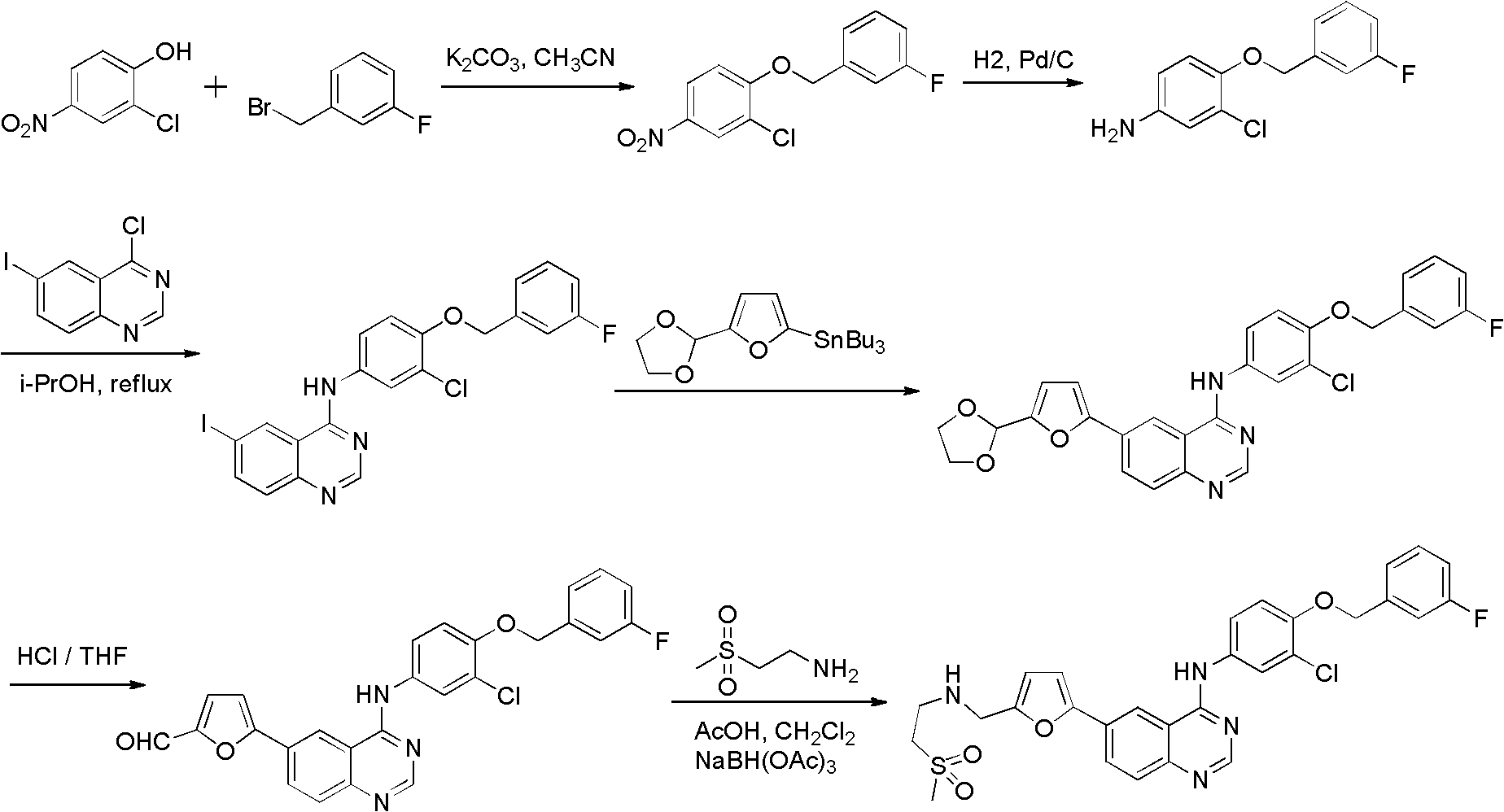
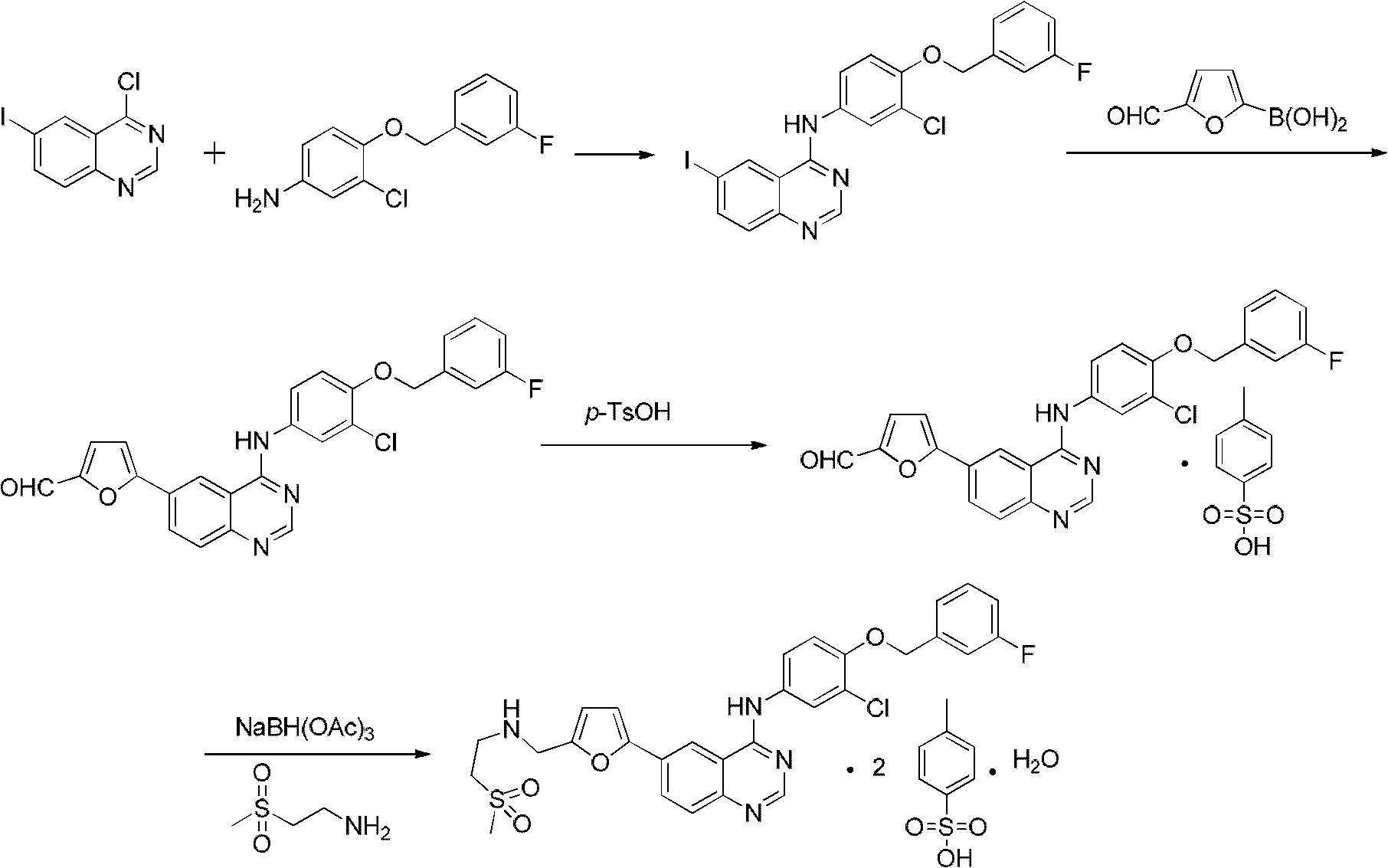
Preparation method of Lapatinib
A lapatinib and compound technology, which is applied in the preparation field of lapatinib, can solve problems to be improved, etc., and achieve the effects of mild reaction conditions and simple process operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Step (1) condensation reaction:
[0092] 2-Amino-5-bromobenzonitrile (100 g, 0.508 mol) and DMF-DMA (72.7 g, 0.610 mol) were stirred and mixed, heated to 40 degrees Celsius for 4 hours, the reaction solution was concentrated to dryness under reduced pressure, and petroleum ether was added 2400ml was stirred and crystallized, filtered, and dried to obtain compound 3 as a solid (110 g, 0.437 mol), with a yield of 86.0%.
[0093] 1 H-NMR (DMSO-d 6 )δ: 3.08(s, 6H), 6.81(d, J=6.57Hz, 1H), 7.50(m, 1H), 7.61(t, J=9.15Hz, 2H).
Embodiment 2
[0095] Step (1) condensation reaction:
[0096] 2-Amino-5-bromobenzonitrile (100 grams, 0.508 moles) and DMF-DMA (180 grams, 1.51 moles) were stirred and mixed, heated to 75 degrees Celsius for 2 hours, the reaction solution was concentrated to dryness under reduced pressure, and a mixed solvent was added 2000 ml (the volume ratio of petroleum ether and methyl tert-butyl ether is 4:1) was stirred for crystallization, filtered, and dried to obtain compound 3 as a solid (116 g, 0.460 mol), with a yield of 90.6%.
Embodiment 3
[0098] Step (1) condensation reaction:
[0099] 2-Amino-5-bromobenzonitrile (100 g, 0.508 mol) and DMF-DMA (300 g, 2.52 mol) were stirred and mixed, heated to 60 degrees Celsius for 1 hour, the reaction solution was concentrated to dryness under reduced pressure, and ethyl 1600 ml of tert-butyl ether was stirred and crystallized, filtered, and dried to obtain compound 3 as a solid (118 g, 0.468 mol), with a yield of 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com