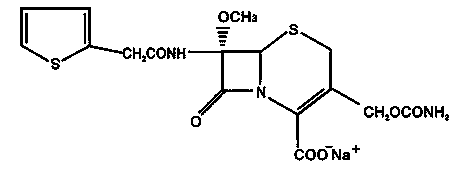
Cefoxitin sodium superfine-powder preparation and preparation method thereof
A technology of cefoxitin sodium and ultrafine powder, which is applied in the field of medicine, can solve the problems of obvious side effects and low purity of active ingredients, and achieve the effect of improving purity, less impurities and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 120 g of 7-α-methoxy-3-deacetyl cephalothin benzathine salt into 1.44 L of acetone, cool to -70 ° C ~ -80 ° C, add 80 g of chlorosulfonyl acetocyanate, and wait for the reaction to When 7-α-methoxy-3-deacetyl cephalothin benzathine salt ≤ 1.5%, it ends; the resulting reaction solution is poured into deionized water, and the hydrolysis reaction is carried out under stirring at a temperature of 0~25°C. In the hydrolyzate Add 1.92L of ethyl acetate, stir for 15 minutes and then filter. After the filtrate is allowed to stand and separate phases, collect the organic phase. Add activated carbon to the above organic phase, stir for 40 minutes and then filter. Concentrate the above filtrate under reduced pressure. When the concentrated juice has crystals Stop concentrating, then raise the temperature to 40°C, add dichloromethane to the concentrated solution at the same time, stir for 40 minutes, and filter until the wet product of cefoxitin acid is obtained.
[0029] Dissol...
Embodiment 2
[0033] Add 120 g of 7-α-methoxy-3-deacetyl cephalothin benzathine salt into 1.44 L of acetone, cool to -70 ° C ~ -80 ° C, add 80 g of chlorosulfonyl acetocyanate, and wait for the reaction to When 7-α-methoxy-3-deacetyl cephalothin benzathine salt ≤ 1.5%, it ends; the resulting reaction solution is poured into deionized water, and the hydrolysis reaction is carried out under stirring at a temperature of 0~25°C. In the hydrolyzate Add 1.92L of ethyl acetate, stir for 15 minutes and then filter. After the filtrate is allowed to stand and separate phases, collect the organic phase. Add activated carbon to the above organic phase, stir for 40 minutes and then filter. Concentrate the above filtrate under reduced pressure. When the concentrated juice has crystals Stop concentrating, then raise the temperature to 40°C, add dichloromethane to the concentrated solution at the same time, stir for 40 minutes, and filter until the wet product of cefoxitin acid is obtained.
[0034] Dissol...
Embodiment 3
[0039] Example 3 - Safety Test (Allergy Test)
[0040] Take 20 white guinea pigs, weighing 280g~350g, male and female. Divided into 4 groups, 5 Meizu, cefoxitin sodium for injection (100mg / mL), ovalbumin solution positive group (30mg / mL), normal saline control group.
[0041]Each group first injected the corresponding solution 0.5mL intraperitoneally every other day, for a total of 3 times, and then divided the animals in the above groups into two groups. One group received cefoxime for injection respectively on the 14th day after the first administration. Butyrate sodium, ovalbumin solution and normal saline 2mL / rat were challenged, and the allergic reaction of the animals after injection was observed, and graded according to the grade standard of allergic reaction. Group 2 was attacked in the same way 21 days after the first injection, and observed. If the grade of allergic reaction is more than 2 grades, the allergic reaction is judged to be positive.
[0042] The resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com