Cefoxitin sodium compound-containing pharmaceutical composition
A technology of cefoxitin sodium and compounds, which is applied in the field of pharmaceutical compounds, can solve the problems affecting the quality of medicines, poor stability of cefoxitin sodium raw materials, large changes in color grade and content, etc., and the method is simple and feasible, and has excellent thermal stability The effect of sex and content is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
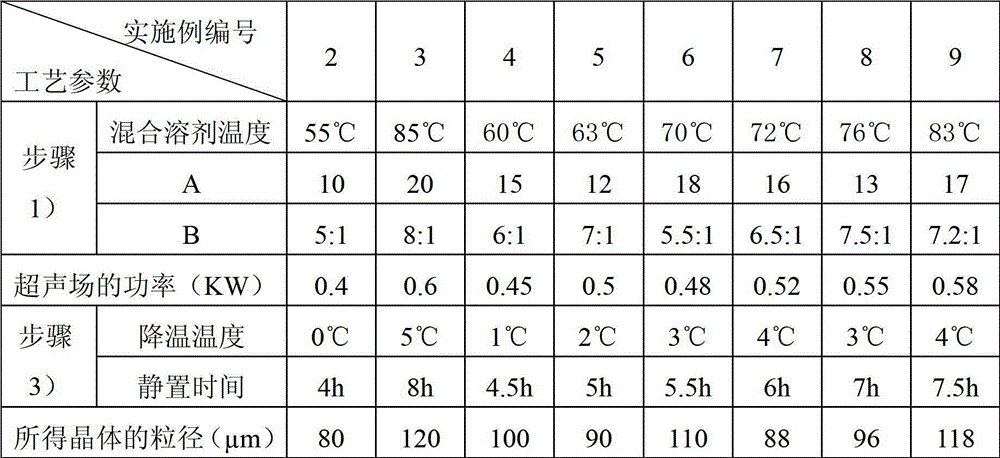
[0045] [Example 1] Preparation of Cefoxitin Sodium Compound Crystal
[0046] 1) adding the crude product of cefoxitin sodium into a mixed solvent of n-butanol and tetrahydrofuran, stirring and dissolving to obtain a solution of cefoxitin sodium in n-butanol and tetrahydrofuran;
[0047] 2) Add diethyl ether dropwise to the n-butanol and tetrahydrofuran solution of cefoxitin sodium obtained in step 1) under an ultrasonic field until crystallization occurs;
[0048] 3) Turn off the ultrasonic field, cool down, let stand, filter, wash the filter cake with ethanol, and dry to obtain the cefoxitin sodium compound crystal.
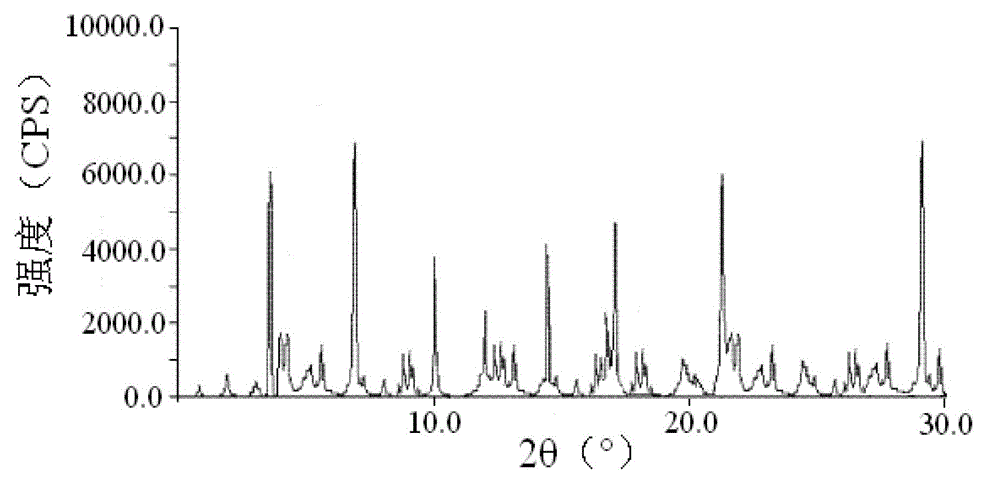
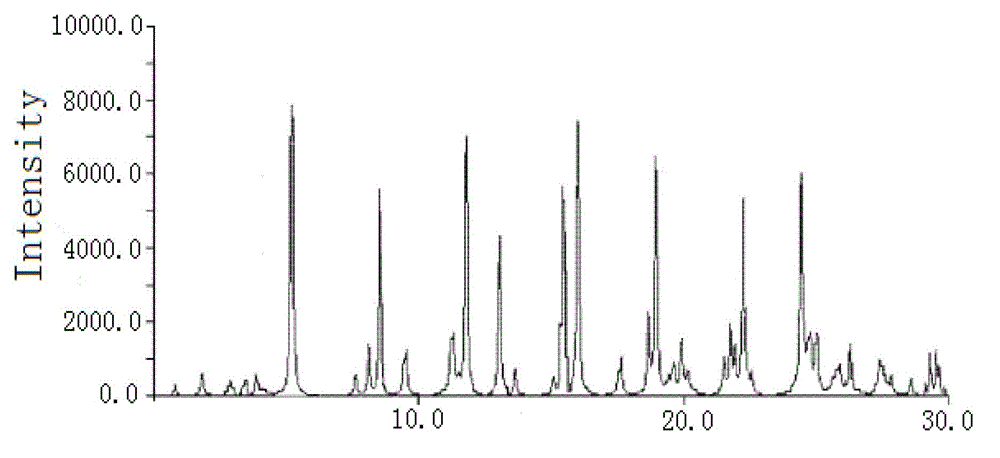
[0049] The particle diameter of the cefoxitin sodium compound crystal of gained is 105 μ m, and this crystal uses Cu-Kα ray to measure, and the X-ray powder diffraction figure that obtains is as follows figure 1 As shown, its X-ray powder diffraction pattern represented by 2θ±0.2° diffraction angle is at 3.50°, 4.00°, 4.25°, 5.50°, 7.00°, 8.75°, 9.00°, 12.00°, ...
preparation Embodiment 1
[0056] [Preparation Example 1] Cefoxitin Sodium Powder Injection
[0057] Dispensing 80 g of cefoxitin sodium compound crystals and 20 g of mannitol into 1000 bottles under aseptic conditions to obtain cefoxitin sodium powder injection.
preparation Embodiment 2
[0058] [Preparation Example 2] Cefoxitin Sodium Powder Injection
[0059] 120 g of cefoxitin sodium compound crystals and 10 g of mannitol are aseptically packed into 1000 bottles to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com