Method for preparing cefoxitin acid
A technology of cefoxitin acid and thiophene acid, which is applied in the field of medicine, can solve the problems of increasing production costs and production costs, and achieve the effects of eliminating low-temperature strong alkali hydrolysis, avoiding side reactions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
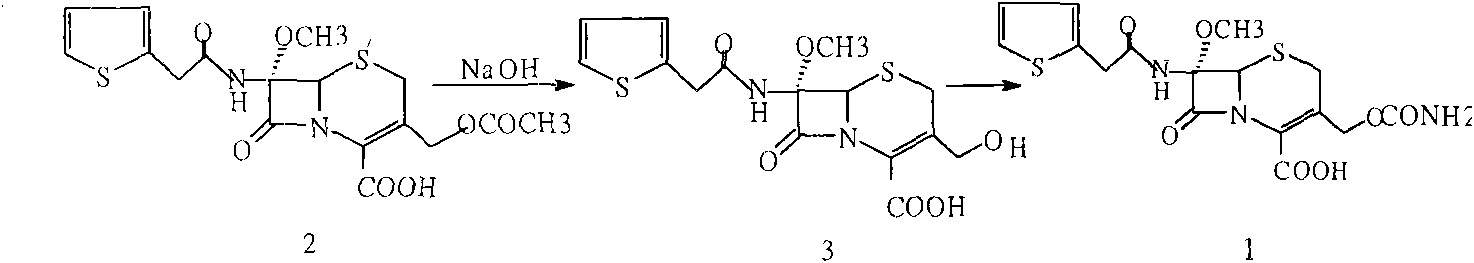
[0026] Embodiment 1: the preparation of methoxycephalosporin (2)
[0027] Add 100g of cefalotin sodium into a mixed solvent of 500ml of dichloromethane and 100ml of methanol, add 15g of methanesulfonic acid and stir to dissolve. After the above reaction solution is completely dissolved, cool the reaction solution to -90°C, add 50ml of dichloromethane dissolved in 30g of N-chlorosuccinimide, keep warm at -90°C, and slowly add dropwise to pre-cool to -10- 450ml of 20% sodium methoxide at -15°C keeps the temperature of the reaction solution stable at -85--90°C, and washes the dropper with 15ml of methanol after dropping. After reacting for 1 hour, add 15g of N-chlorosuccinimide, continue to react for 2 hours, add 21g of stopper, continue to react for 10 minutes, add 150g of 80% acetic acid at -75-78°C, and use 10% of chlorine respectively Wash 3 times with 500 ml of sodium chloride solution, extract the aqueous phase with 200 ml of dichloromethane and combine the organic layers....
Embodiment 2
[0028] Embodiment 2: contrast (original technology)
[0029] Add 10% sodium carbonate solution to the dichloromethane layer obtained in Implementation 1, adjust to PH9-10, separate the organic layer, wash with a small amount of purified water, combine the water layer, add 100ml of methanol, cool to -40°C, add 30% 110ml of sodium hydroxide, reacted at -30-35°C for 1 hour, added 100ml of 80% acetic acid to adjust the pH to 4-5, precipitated solid, continued to stir for 30 minutes, filtered, dried until the water content was less than 5‰, and obtained 3 -Hydroxymethyl cephalosporin 68g (compound (3)) content 99.2% (normalized method).
Embodiment 3
[0030] Embodiment 3. contrast (original technology)
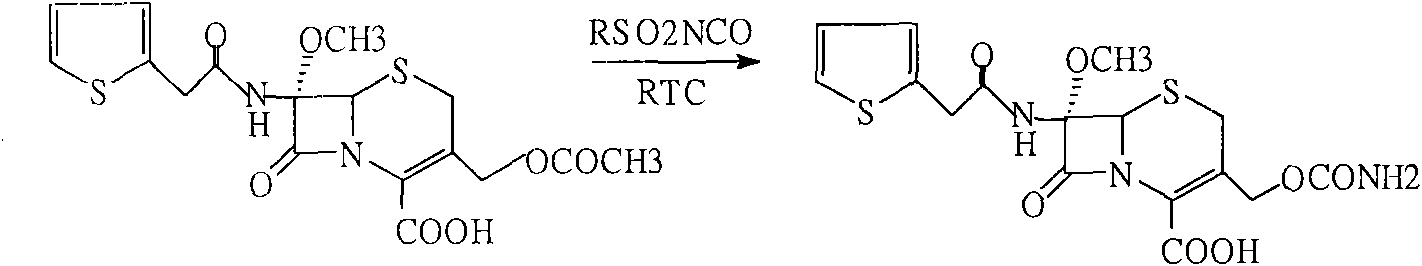
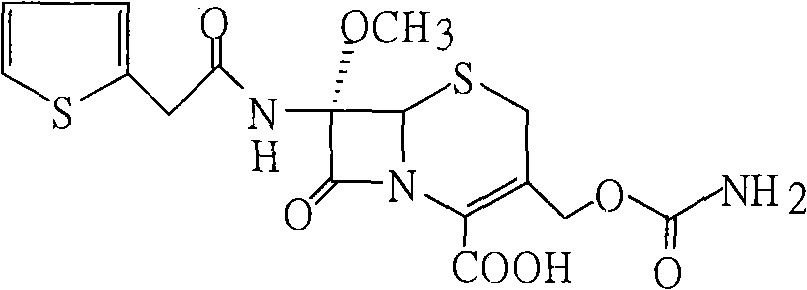
[0031] Add 60g of compound (3) into 200ml tetrahydrofuran, stir to dissolve, cool to -60°C, add 50g isocyanate chlorosulfonate, react at -40-45°C for 1 hour, add 10% sodium chloride solution, hydrolyze 1 Hours, keep warm at 23-25°C, add diethyl hydrochloric acid dropwise to ph=1.3-1.5, precipitate solid particles, grow crystals for 30 minutes, cool to 5°C, filter after 30 minutes, and wash with DM. Dry until the water content is less than 3‰ to obtain 54 g of cefoxitin acid. Content: 99.3% (normalized method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com