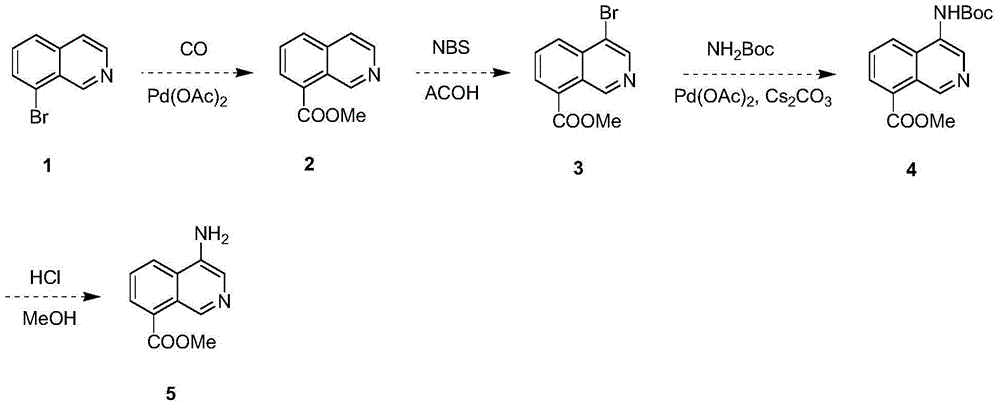
Synthesis method of 4-aminoisoquinoline-8-methyl formate
A technology of aminoisoquinoline and isoquinolinecarboxylic acid, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of poor selectivity of nitration reaction, difficulty in large-scale production, difficulty in separation, etc., and achieves convenient operation, simple post-treatment, and good selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] The synthetic method of 4-aminoisoquinoline-8-methyl carboxylate, technique is as follows:
[0017] The first step: the synthesis of methyl 8-isoquinolinecarboxylate
[0018] 5 g of palladium acetate was added to a solution of 8-bromoisoquinoline (104 g, 500 mmol) in methanol (1000 mL), and the reaction system was replaced with CO three times and reacted at 60° C. and 60 psi pressure for 8 h. After the mixture was evaporated to dryness, 500 mL of water and 1000 mL of dichloromethane were added. After the aqueous phase was separated, the organic layer was spin-dried, and the residue was washed with petroleum ether to obtain methyl 8-isoquinolinecarboxylate (90 g, 96%).
[0019] The second step: the synthesis of methyl 4-bromoisoquinoline-8-carboxylate
[0020] At 110°C, N-bromosuccinimide (57.0 g, 321 mmol) was added to a solution of methyl 8-isoquinolinecarboxylate (50 g, 267 mmol) in acetic acid (1000 mL), and the mixture was heated to reflux. After reacting for 16 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com