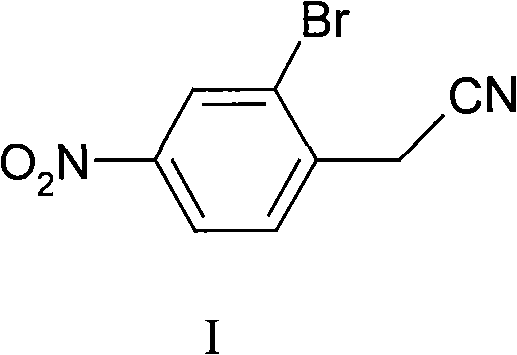
Synthesis of 2-bromine-4-nitrobenzene ethane nitrile
A technology of nitrophenylacetonitrile and p-nitrophenylacetonitrile, applied in the field of synthesis of 2-bromo-4 nitrophenylacetonitrile, achieving the effects of less energy consumption, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
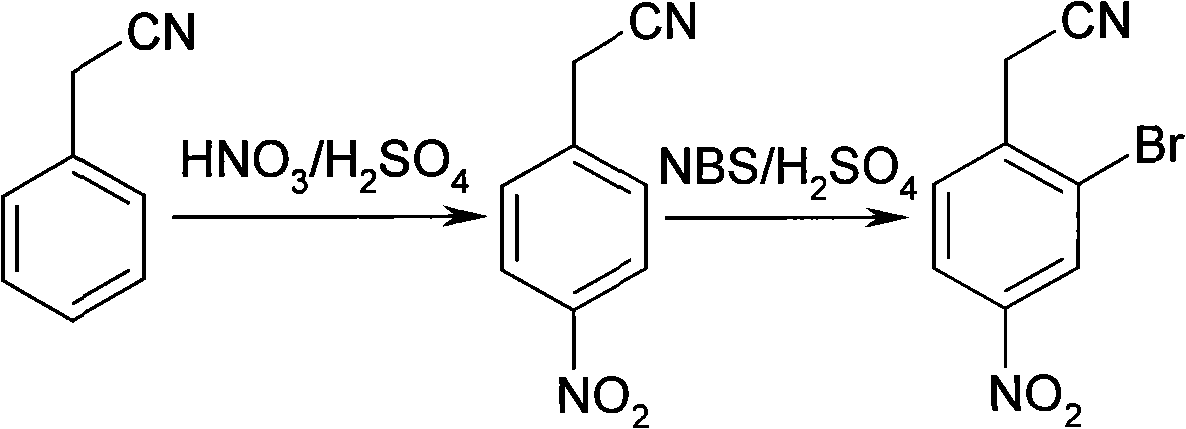
[0015] Embodiment 1, a kind of synthetic method of 2-bromo-4-nitrophenylacetonitrile, take phenylacetonitrile as main raw material, successively through the following steps:
[0016] 1) Nitration: Add 178.4mL of concentrated nitric acid and 267.2mL of concentrated sulfuric acid to a 1000mL three-neck flask, cool in an ice-water bath to 5°C, add 64mL of phenylacetonitrile dropwise, and control the temperature not to exceed 10°C. After the addition, remove the ice-water bath and warm up slowly Continue the reaction at room temperature (25° C.) for 2 h, then pour the obtained reaction product into 2500 g of ice water with stirring to produce a light yellow solid, which is filtered to obtain a filter cake. The filter cake was washed with water until neutral, and dried to obtain 62.5 g of p-nitrophenylacetonitrile, mp: 114-115°C, yield 69.1%.
[0017] 2) Bromination: Add 50.0 mL of concentrated sulfuric acid to a 250 mL three-necked flask, cool to -20°C with a low-temperature therm...
Embodiment 2
[0020] Embodiment 2, a kind of synthetic method of 2-bromo-4-nitrophenylacetonitrile, take phenylacetonitrile as main raw material, successively through the following steps:
[0021] 1) Nitration: Add 74.4mL of concentrated nitric acid and 372.0mL of concentrated sulfuric acid to a 1000mL three-neck flask, add 64mL of phenylacetonitrile dropwise at room temperature (25°C), control the temperature not to exceed 30°C, and continue the reaction at room temperature (25°C) after the addition After 3 hours, the resulting reaction product was poured into 1000 g of ice water with stirring, and a light yellow solid was produced immediately, which was filtered to obtain a filter cake. The filter cake was washed with water until neutral, and dried to obtain 41.3 g of p-nitrophenylacetonitrile, mp: 114-115°C, yield 45.7%.
[0022] 2) Bromination: In a 250mL three-necked flask, add 88.0mL of concentrated sulfuric acid, cool in an ice-water bath to -20°C, add 16.2g of p-nitrophenylacetonitr...
Embodiment 3
[0024] Embodiment 3, a kind of synthetic method of 2-bromo-4-nitrophenylacetonitrile, take phenylacetonitrile as main raw material, successively through the following steps:
[0025] 1) Nitration: Add 186.1mL of concentrated nitric acid and 186.1mL of concentrated sulfuric acid to a 1000mL three-neck flask, and control the reaction temperature at -20°C in a low-temperature thermostat; add 64mL of phenylacetonitrile dropwise, and control the temperature not to exceed -10°C. After the addition- The reaction was continued at 20°C for 0.5 h; then the obtained reaction product was poured into 5000 g of ice water with stirring, and a pale yellow solid was formed immediately, which was filtered to obtain a filter cake. The filter cake was washed with water until neutral, and dried to obtain 59.6 g of p-nitrophenylacetonitrile, mp: 114-115°C, yield 65.9%.
[0026] 2) Bromination: In a 250mL three-necked flask, add 26.4mL of concentrated sulfuric acid, add 16.2g of p-nitrophenylacetoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com