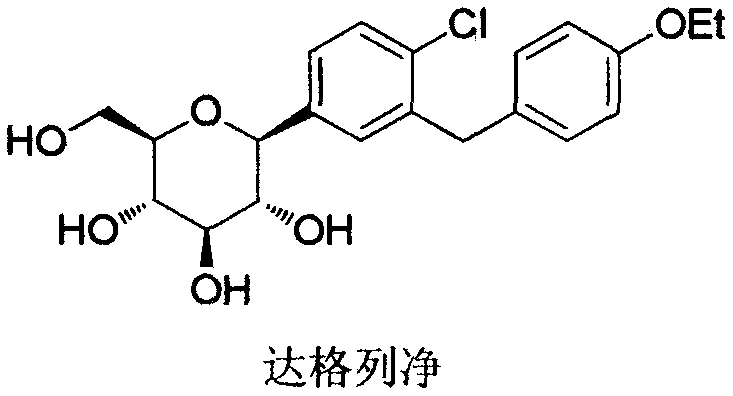
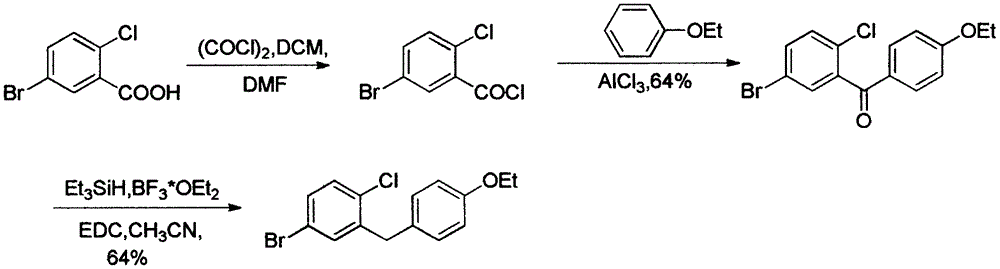
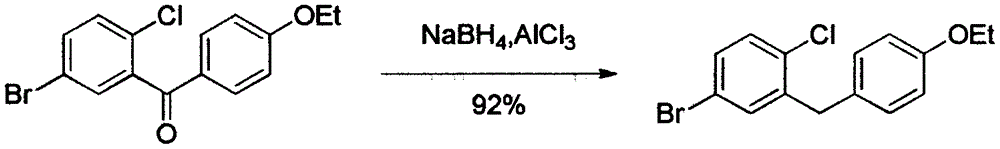Preparation method of 5-bromine-2-chlorine-4'-ethyoxyl diphenylmethane
A technology of chlorotoluene and synthetic methods, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as post-processing difficulties, and achieve the effects of easy post-processing, mild reaction conditions, and rich synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be further described below through the examples, but the examples do not limit the protection scope of the present invention.
[0025] (1) Preparation of 4-bromo-2-methylaniline
[0026] Place 8.1ml (75.4mmol) of o-toluidine in a dry 250ml single-necked bottle, and stir it magnetically under an ice-water bath. Dissolve 13.5g (75.8mmol) of N-bromosuccinimide in 60ml of N,N-dimethylformamide, place it in a dropping funnel and drop it in slowly, keep the reaction liquid at about 0°C, and add it for 5 hours . The reaction solution was poured into 600ml of ice water, the solid was precipitated, and the solid was obtained by suction filtration. The aqueous phase was extracted with 50ml of ethyl acetate and concentrated to obtain a small amount of solid. The combined solid was washed with petroleum ether (25ml*3) to obtain 13.2g of solid. The yield was 95%, and the product was directly used in the next reaction without purification. m.p.50-52℃. 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com